Novel Tools to Assess Muscle Sarcopenic Process in ICU Patients: Are They Worthwhile?
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 2020, 46, 637. [Google Scholar] [CrossRef]
- Latronico, N.; Herridge, M.; Hopkins, R.O.; Angus, D.; Hart, N.; Hermans, G.; Iwashyna, T.; Arabi, Y.; Citerio, G.; Ely, E.W.; et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017, 43, 1270. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The rate and assessment of muscle wasting during critical illness: A systematic review and meta-analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef]
- Zayed, Y.; Kheiri, B.; Barbarawi, M.; Chahine, A.; Rashdan, L.; Chintalapati, S.; Bachuwa, G.; Al-Sanouri, I. Effects of neuromuscular electrical stimulation in critically ill patients: A systematic review and meta-analysis of randomised controlled trials. Aust. Crit. Care 2020, 33, 203. [Google Scholar] [CrossRef]
- Verceles, A.C.; Serra, M.; Davis, D.; Alon, G.; Wells, C.L.; Parker, E.; Sorkin, J.; Bhatti, W.; Terrin, M.L. Combining exercise, protein supplementation and electric stimulation to mitigate muscle wasting and improve outcomes for survivors of critical illness—The ExPrES study. Heart Lung 2023, 58, 229. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591, Erratum in JAMA 2014, 311, 625. Padhke, Rahul [corrected to Phadke, Rahul]. [Google Scholar] [CrossRef]
- Maislin, G.; Ahmed, M.M.; Gooneratne, N.; Thorne-Fitzgerald, M.; Kim, C.; Teff, K.; Arnardottir, E.S.; Benediktsdottir, B.; Einarsdottir, H.; Juliusson, S.; et al. Single slice vs. volumetric MR assessment of visceral adipose tissue: Reliability and validity among the overweight and obese. Obesity 2012, 20, 2124. [Google Scholar] [CrossRef]
- Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Twisk, J.W.; Oudemans-van Straaten, H.M.; Weijs, P.J. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef]
- Kim, D.; Sun, J.S.; Lee, Y.H.; Lee, J.H.; Hong, J.; Lee, J.M. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin. Nutr. 2019, 38, 2747. [Google Scholar] [CrossRef]
- Lee, Y.I.; Ko, R.E.; Ahn, J.; Carriere, K.C.; Ryu, J.A. Association between neurologic outcomes and changes of muscle mass measured by brain computed tomography in neurocritically ill patients. J. Clin. Med. 2021, 11, 90. [Google Scholar] [CrossRef]
- Diallo, T.D.; Rospleszcz, S.; Fabian, J.; Walter, S.S.; Maurer, E.; Storz, C.; Roemer, F.; Rathmann, W.; Peters, A.; Jungmann, P.M.; et al. Associations of myosteatosis with disc degeneration: A 3T magnetic resonance imaging study in individuals with impaired glycaemia. J. Cachexia Sarcopenia Muscle 2023. epub ahead of print. [Google Scholar] [CrossRef]
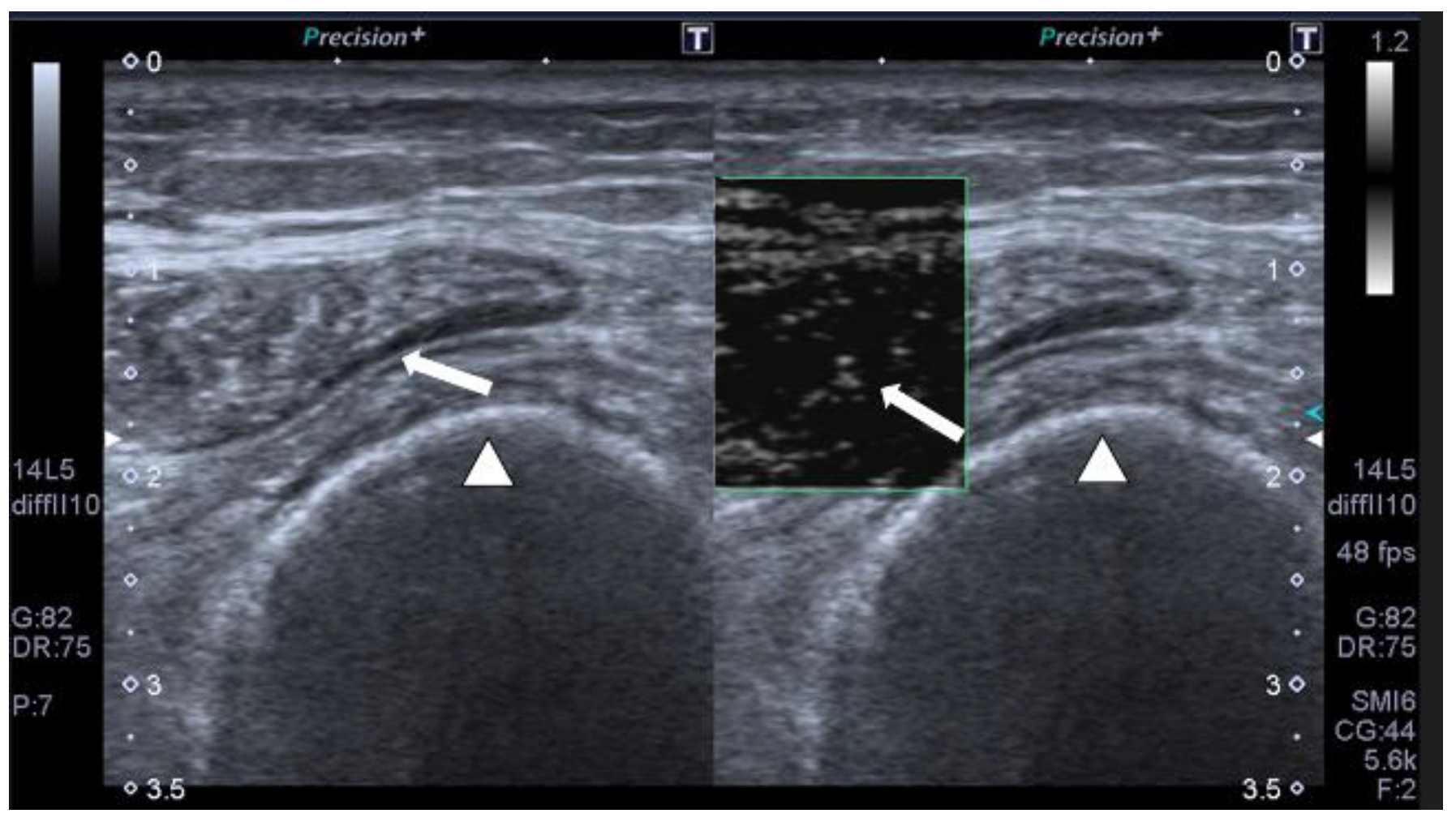
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of muscle wasting in long-stay ICU patients using a new ultrasound protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef]
- Formenti, P.; Umbrello, M.; Coppola, S.; Froio, S.; Chiumello, D. Clinical review: Peripheral muscular ultrasound in the ICU. Ann. Intensive Care 2019, 9, 57. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; di Mario, F.; Ciuni, A.; Palumbo, A.; Peyronel, F.; Maggiore, U.; Fiaccadori, E. Validation by CT scan of quadriceps muscle thickness measurement by ultrasound in acute kidney injury. J. Nephrol. 2020, 33, 109. [Google Scholar] [CrossRef]
- Giovannini, S.; Brau, F.; Forino, R.; Berti, A.; D’Ignazio, F.; Loreti, C.; Bellieni, A.; D’Angelo, E.; Di Caro, F.; Biscotti, L.; et al. Sarcopenia: Diagnosis and management, state of the art and contribution of ultrasound. J. Clin. Med. 2021, 10, 5552. [Google Scholar] [CrossRef]
- Deng, M.; Zhou, X.; Li, Y.; Yin, Y.; Liang, C.; Zhang, Q.; Lu, J.; Wang, M.; Wang, Y.; Sun, Y.; et al. Ultrasonic elastography of the rectus femoris, a potential tool to predict sarcopenia in patients with chronic obstructive pulmonary disease. Front. Physiol. 2022, 12, 783421. [Google Scholar] [CrossRef]
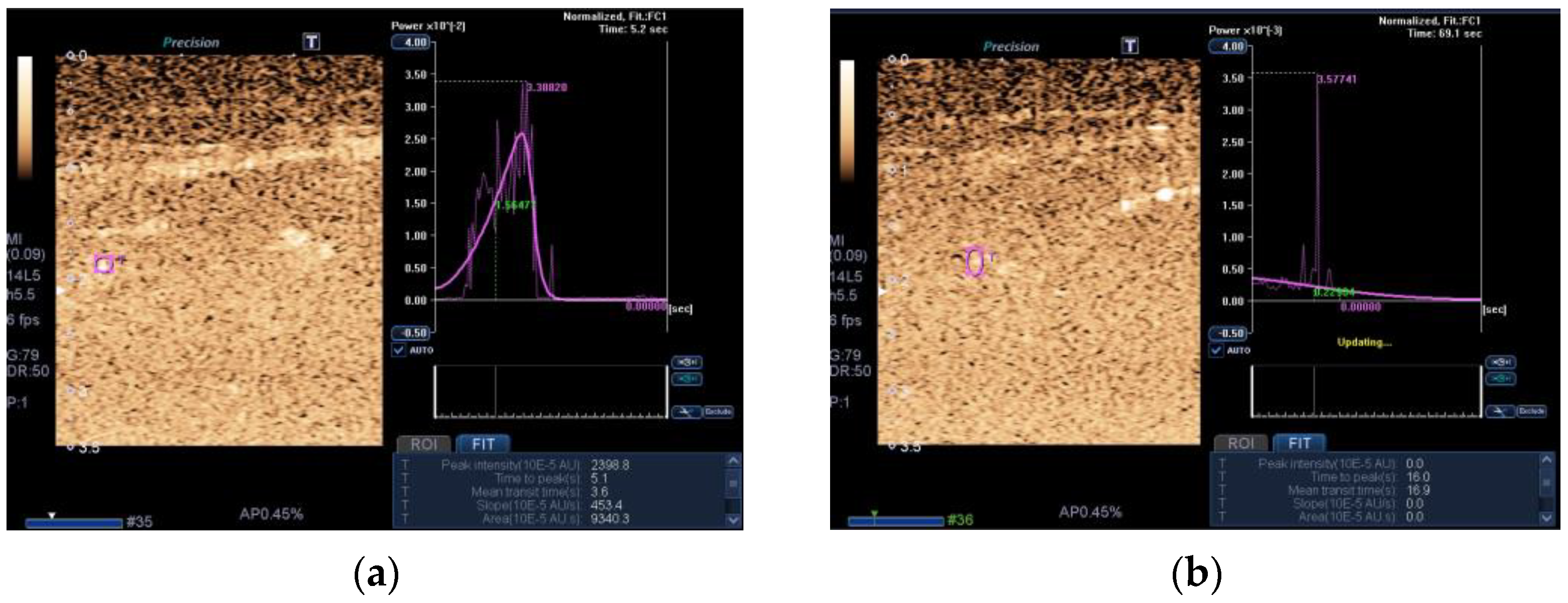
- Jones, E.J.; Atherton, P.J.; Piasecki, M.; Phillips, B.E. Contrast-enhanced ultrasound repeatability for the measurement of skeletal muscle microvascular blood flow. Exp. Physiol. 2023, 108, 549. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 2019, 31, 1755. [Google Scholar] [CrossRef]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Lübbe-Vazquez, F.; Ruiz-Santana, S. Novel high-quality sonographic methods to diagnose muscle wasting in long-stay critically ill patients: Shear wave elastography, superb microvascular imaging and contrast-enhanced ultrasound. Nutrients 2021, 13, 2224. [Google Scholar] [CrossRef]
- Soilemezi, E.; Savvidou, S.; Sotiriou, P.; Smyrniotis, D.; Tsagourias, M.; Matamis, D. Tissue doppler imaging of the diaphragm in healthy subjects and critically ill patients. Am. J. Respir. Crit. Care Med. 2020, 202, 1005. [Google Scholar] [CrossRef]
- Aarab., Y.; Flatres, A.; Garnier, F.; Capdevila, M.; Raynaud, F.; Lacampagne, A.; Chapeau, D.; Klouche, K.; Etienne, P.; Jaber, S.; et al. Shear wave elastography, a new tool for diaphragmatic qualitative assessment: A translational study. Am. J. Respir. Crit. Care Med. 2021, 204, 797. [Google Scholar] [CrossRef]
- Flatres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S.; et al. Real-time shear wave ultrasound elastography: A new tool for the evaluation of diaphragm and limb muscle stiffness in critically ill patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef]
- TEAM study investigators and the ANZICS clinical trials group; Hodgson, C.L.; Bailey, M.; Bellomo, R.; Brickell, K.; Broadley, T.; Buhr, H.; Gabbe, B.J. Early active mobilization during mechanical ventilation in the ICU. N. Engl. J. Med. 2022, 387, 1747. [Google Scholar] [CrossRef]
- Viana, M.V.; Becce, F.; Pantet, O.; Schmidt, S.; Bagnoud, G.; Thaden, J.J.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Voidey, A.; Deutz, N.E.P.; et al. Impact of β-hydroxy-β-methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: A RCT. Clin. Nutr. 2021, 40, 4878. [Google Scholar] [CrossRef]
- Supinski, G.S.; Netzel, P.F.; Westgate, P.M.; Schroder, E.A.; Wang, L.; Callahan, L.A. A randomized controlled trial to determine whether beta-hydroxy-beta-methylbutyrate and/or eicosapentaenoic acid improves diaphragm and quadriceps strength in critically Ill mechanically ventilated patients. Crit. Care. 2021, 25, 308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Santana, S.; Hernández-Socorro, C.R. Novel Tools to Assess Muscle Sarcopenic Process in ICU Patients: Are They Worthwhile? J. Clin. Med. 2023, 12, 3473. https://doi.org/10.3390/jcm12103473
Ruiz-Santana S, Hernández-Socorro CR. Novel Tools to Assess Muscle Sarcopenic Process in ICU Patients: Are They Worthwhile? Journal of Clinical Medicine. 2023; 12(10):3473. https://doi.org/10.3390/jcm12103473
Chicago/Turabian StyleRuiz-Santana, Sergio, and Carmen Rosa Hernández-Socorro. 2023. "Novel Tools to Assess Muscle Sarcopenic Process in ICU Patients: Are They Worthwhile?" Journal of Clinical Medicine 12, no. 10: 3473. https://doi.org/10.3390/jcm12103473
APA StyleRuiz-Santana, S., & Hernández-Socorro, C. R. (2023). Novel Tools to Assess Muscle Sarcopenic Process in ICU Patients: Are They Worthwhile? Journal of Clinical Medicine, 12(10), 3473. https://doi.org/10.3390/jcm12103473





