Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
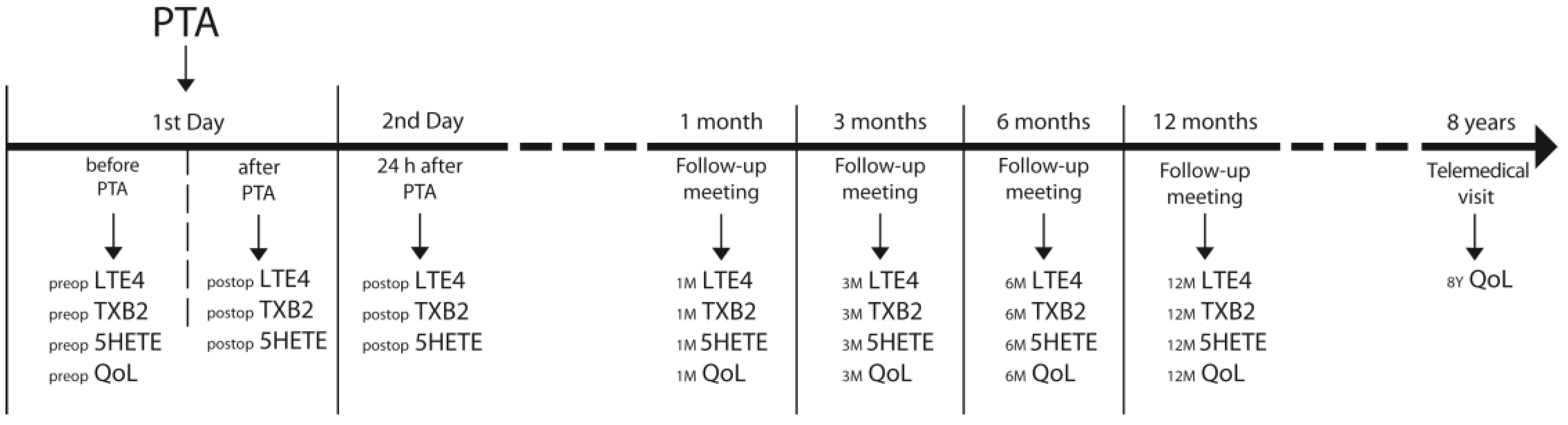
2.2. Follow-Up Meetings Outcomes Assessment
2.3. Laboratory Measurements
2.4. Quality-of-Life Assessment
2.5. Statistical Analysis
2.6. Ethical Aspects
3. Results
3.1. Patients Characteristics
3.2. Quality of Life and Its Changes after Endovascular Procedures
3.3. Preoperative Inflammatory Biomarkers and Life Quality
3.4. Inflammatory Biomarkers and Life Quality during Follow-Up
3.5. Impact of Changes in Inflammatory Biomarkers Concentrations on Quality-of-Life Changes during Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, J.; Santulli, G. Update on Peripheral Artery Disease: Epidemiology and Evidence-Based Facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, Regional, and National Prevalence and Risk Factors for Peripheral Artery Disease in 2015: An Updated Systematic Review and Analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Guralnik, J.M.; Tian, L.; Kibbe, M.R.; Ferrucci, L.; Zhao, L.; Liu, K.; Liao, Y.; Gao, Y.; Criqui, M.H. Incidence and Prognostic Significance of Depressive Symptoms in Peripheral Artery Disease. J. Am. Heart Assoc. 2015, 5, e002959. [Google Scholar] [CrossRef]
- Sliwka, A.; Furgal, M.; Maga, P.; Drelicharz, L.; Mika, P.; WŁoch, T.; Nowobilski, R. The Role of Psychopathology in Perceiving, Reporting and Treating Intermittent Claudication: A Systematic Review. Int. Angiol. 2018, 37, 335–345. [Google Scholar] [CrossRef]
- Brostow, D.P.; Petrik, M.L.; Starosta, A.J.; Waldo, S.W. Depression in Patients with Peripheral Arterial Disease: A Systematic Review. Eur. J. Cardiovasc. Nurs. 2017, 16, 181–193. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Saravanan, A.; Bajaj, P.; Mathews, H.L.; Tell, D.; Starkweather, A.; Janusek, L. Behavioral Symptom Clusters, Inflammation, and Quality of Life in Chronic Low Back Pain. Pain Manag. Nurs. 2021, 22, 361–368. [Google Scholar] [CrossRef]
- Biernacki, W.A.; Kharitonov, S.A.; Biernacka, H.M.; Barnes, P.J. Effect of Montelukast on Exhaled Leukotrienes and Quality of Life in Asthmatic Patients. Chest 2005, 128, 1958–1963. [Google Scholar] [CrossRef]
- Fredman, G.; MacNamara, K.C. Atherosclerosis Is a Major Human Killer and Non-Resolving Inflammation Is a Prime Suspect. Cardiovasc. Res. 2021, 117, 2563–2574. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and Its Resolution in Atherosclerosis: Mediators and Therapeutic Opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. Inflammation Revisited: Atherosclerosis in the Post-CANTOS Era. Eur. Cardiol. Rev. 2017, 12, 89–91. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Yates, D.P.; Kramer, C.M.; Feller, A.; Mahling, P.; Colin, L.; Clough, T.; Wang, T.; LaPerna, L.; Patel, A.; et al. A Randomized, Placebo-Controlled Trial of Canakinumab in Patients with Peripheral Artery Disease. Vasc. Med. 2019, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann-Maga, A.; Kaszuba, M.; Maga, M.; Włodarczyk, A.; Krężel, J.; Kaczmarczyk, P.; Bogucka, K.; Maga, P. Leukotrienes in the Atherosclerotic Cardiovascular Diseases—A Systematic Review. Acta Angiol. 2022, 28, 147–153. [Google Scholar] [CrossRef]
- Bäck, M. Leukotriene Receptors: Crucial Components in Vascular Inflammation. Sci. World J. 2007, 7, 1422–1439. [Google Scholar] [CrossRef]
- Maga, P.; Sanak, M.; Rewerska, B.; Maga, M.; Jawien, J.; Wachsmann, A.; Rewerski, P.; Szczeklik, W. Urinary Cysteinyl Leukotrienes in One-Year Follow-up of Percutaneous Transluminal Angioplasty for Peripheral Arterial Occlusive Disease. Atherosclerosis 2016, 249, 174–180. [Google Scholar] [CrossRef]
- Wachsmann-Maga, A.; Włodarczyk, A.; Maga, M.; Batko, K.; Bogucka, K.; Kapusta, M.; Terlecki, P.; Maga, P. Leukotrienes E4 and B4 and Vascular Endothelium—New Insight into the Link between Vascular Inflammation and Peripheral Arterial Disease and Its Complications. Cells 2023. Epub ahead of printing. [Google Scholar] [CrossRef]
- Maga, P.; Sanak, M.; Jawien, J.; Rewerska, B.; Maga, M.; Wachsmann, A.; Koziej, M.; Gregorczyk-Maga, I.; Nizankowski, R. 11-Dehydro Thromboxane B2 Levels after Percutaneous Transluminal Angioplasty in Patients with Peripheral Arterial Occlusive Disease during a One Year Follow-up Period. J. Physiol. Pharmacol. 2016, 67, 377–383. [Google Scholar]
- Belowski, A.; Partyka, Ł.; Krzanowski, M.; Polczyk, R.; Maga, P.; Maga, M.; Acquadro, C.; Lambe, J.; Morgan, M.; Niżankowski, R. Clinical and Linguistic Validation of the Polish Version of VascuQol: A Disease-Specific Quality-of-Life Questionnaire Assessing Patients with Chronic Limb Ischemia. Pol. Arch. Intern. Med. 2019, 129, 167–174. [Google Scholar] [CrossRef]
- Nordanstig, J.; Wann-Hansson, C.; Karlsson, J.; Lundström, M.; Pettersson, M.; Morgan, M.B.F. Vascular Quality of Life Questionnaire-6 Facilitates Health-Related Quality of Life Assessment in Peripheral Arterial Disease. J. Vasc. Surg. 2014, 59, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Yang, L.S.; Tew, M.; Westcott, M.J.; Spelman, T.D.; Choong, P.F.; Davies, A.H. Quality of Life in Chronic Limb Threatening Ischaemia: Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Thanigaimani, S.; Phie, J.; Sharma, C.; Wong, S.; Ibrahim, M.; Huynh, P.; Moxon, J.; Jones, R.; Golledge, J. Network Meta-Analysis Comparing the Outcomes of Treatments for Intermittent Claudication Tested in Randomized Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019672. [Google Scholar] [CrossRef] [PubMed]
- Kumlin, M. Measurement of Leukotrienes in Humans. Am. J. Respir. Crit. Care Med. 2000, 161, S102–S106. [Google Scholar] [CrossRef]
- Piper, K.; Garelnabi, M. Eicosanoids: Atherosclerosis and Cardiometabolic Health. J. Clin. Transl. Endocrinol. 2020, 19, 100216. [Google Scholar] [CrossRef]
- Murphy, R.C.; Gijón, M.A. Biosynthesis and Metabolism of Leukotrienes. Biochem. J. 2007, 405, 379–395. [Google Scholar] [CrossRef]
- Rabinovitch, N. Urinary Leukotriene E4. Immunol. Allergy Clin. N. Am. 2007, 27, 651–664. [Google Scholar] [CrossRef]
- Mayatepek, E.; Hoffmann, G.F. Leukotrienes: Biosynthesis, Metabolism, and Pathophysiologic Significance. Pediatr. Res. 1995, 37, 55–60. [Google Scholar] [CrossRef]
- Ingelsson, E.; Yin, L.; Bäck, M. Nationwide Cohort Study of the Leukotriene Receptor Antagonist Montelukast and Incident or Recurrent Cardiovascular Disease. J. Allergy Clin. Immunol. 2012, 129, 702–707.e2. [Google Scholar] [CrossRef]
- Nowakowski, A.C.H.; Graves, K.Y.; Sumerau, J.E. Mediation Analysis of Relationships between Chronic Inflammation and Quality of Life in Older Adults. Health Qual. Life Outcomes 2016, 14, 46. [Google Scholar] [CrossRef]
- Nowakowski, A.C.H. Chronic Inflammation and Quality of Life in Older Adults: A Cross-Sectional Study Using Biomarkers to Predict Emotional and Relational Outcomes. Health Qual. Life Outcomes 2014, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Choy, E.H. C-Reactive Protein and Implications in Rheumatoid Arthritis and Associated Comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.; Pétré, B.; Schleich, F.; Zahraei, H.N.; Donneau, A.F.; Silvestre, A.; Henket, M.; Paulus, V.; Guissard, F.; Guillaume, M.; et al. Predictors of Asthma-Related Quality of Life in a Large Cohort of Asthmatics: A Cross-Sectional Study in a Secondary Care Center. Clin. Transl. Allergy 2021, 11, e12054. [Google Scholar] [CrossRef] [PubMed]
- Lodin, K.; Lekander, M.; Syk, J.; Alving, K.; Petrovic, P.; Andreasson, A. Longitudinal Co-Variations between Inflammatory Cytokines, Lung Function and Patient Reported Outcomes in Patients with Asthma. PLoS ONE 2017, 12, e0185019. [Google Scholar] [CrossRef] [PubMed]
- Afzal, N.A.; Van Der Zaag-Loonen, H.J.; Arnaud-Battandier, F.; Davies, S.; Murch, S.; Derkx, B.; Heuschkel, R.; Fell, J.M. Improvement in Quality of Life of Children with Acute Crohn’s Disease Does Not Parallel Mucosal Healing after Treatment with Exclusive Enteral Nutrition. Aliment. Pharmacol. Ther. 2004, 20, 167–172. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of Life in Inflammatory Bowel Disease in Remission: The Impact of IBS-like Symptoms and Associated Psychological Factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef]
- Peters, K.M.; Carrico, D.J.; Diokno, A.C. Characterization of a Clinical Cohort of 87 Women with Interstitial Cystitis/Painful Bladder Syndrome. Urology 2008, 71, 634–640. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Maydych, V. The Interplay between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Faugere, M.; Micoulaud-Franchi, J.A.; Faget-Agius, C.; Lançon, C.; Cermolacce, M.; Richieri, R. Quality of Life Is Associated with Chronic Inflammation in Depression: A Cross-Sectional Study. J. Affect. Disord. 2018, 227, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chan, S.L.; Mo, F.; Hui, E.P.; Koh, J.; Chan, A.K.; Tang, N.L.; Chu, C.M.; Hui, J.; Lee, K.F.; et al. Status of Inflammation in Relation to Health Related Quality of Life in Hepatocellular Carcinoma Patients. Qual. Life Res. 2019, 28, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Roediger, M.; De Fátima Nunes Marucci, M.; Duim, E.L.; Santos, J.L.F.; De Oliveira Duarte, Y.A.; De Oliveira, C. Inflammation and Quality of Life in Later Life: Findings from the Health, Well-Being and Aging Study (SABE). Health Qual. Life Outcomes 2019, 17, 26. [Google Scholar] [CrossRef]
- Linn, Y.L.; Choke, E.T.C.; Yap, C.J.Q.; Tan, R.Y.; Patel, A.; Tang, T.Y. Utility of Sirolimus Coated Balloons in the Peripheral Vasculature—A Review of the Current Literature. CVIR Endovasc. 2022, 5, 29. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on Peripheral Arterial Disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef]
- Cerda, A.; Pavez, M.; Manriquez, V.; Luchessi, A.D.; Leal, P.; Benavente, F.; Fajardo, C.M.; Salazar, L.; Hirata, M.H.; Hirata, R.D.C. Effects of Clopidogrel on Inflammatory Cytokines and Adhesion Molecules in Human Endothelial Cells: Role of Nitric Oxide Mediating Pleiotropic Effects. Cardiovasc. Ther. 2017, 35, e12261. [Google Scholar] [CrossRef]
| Patients Characteristics | ||
| Participants (n, %) | 175 | 100% |
| Age (mean, SD) | 64.77 | ±8.94 |
| Male (n, %) | 120 | 68.57% |
| Active smoking habit (n, %) | 47 | 26.86% |
| Former smokers (n, %) | 106 | 60.57% |
| Comorbidities | ||
| Coronary artery disease (n, %) | 77 | 44.25% |
| Diabetes mellitus t II (n, %) | 116 | 66.29% |
| Hypertension (n, %) | 128 | 73.14% |
| Hypercholesterolemia (n, %) | 73 | 41.71% |
| Heart failure (n, %) | 21 | 12.00% |
| History of MI (n, %) | 47 | 26.86% |
| History of stroke (n, %) | 17 | 9.71% |
| Peripheral Interventions History | ||
| No | 88 | 50.29% |
| Yes, within 1 limb (n, %) | 49 | 28.0% |
| Yes, within both limbs (n, %) | 38 | 21.71% |
| More than 1 restenosis (n, %) | 32 | 18.29% |
| VascuQol Preoperative | VascuQol 1 M | VascuQol 3 M | VascuQol 6 M | VascuQol 12 M | VascuQol 8 Y | ||
|---|---|---|---|---|---|---|---|
| LTE4 | Preoperative | −0.46 ** | −0.25 ** | −0.26 ** | −0.23 ** | −0.22 ** | 0.16 |
| 1 M FU | −0.32 ** | −0.55 ** | −0.44 ** | −0.23 ** | −0.24 ** | 0.08 | |
| 3 M FU | −0.35 ** | −0.37 ** | −0.72 ** | −0.41 ** | −0.26 ** | 0.06 | |
| 6 M FU | −0.30 ** | −0.24 ** | −0.42 ** | −0.57 ** | −0.31 ** | 0.11 | |
| 12 M FU | −0.28 ** | −0.18 * | −0.33 ** | −0.37 ** | −0.71 ** | 0.03 | |
| TXB2 | Preoperative | −0.52 ** | −0.15 * | −0.24 ** | −0.24 ** | −0.20 ** | 0.10 |
| 1 M FU | −0.24 ** | −0.21 ** | −0.32 ** | −0.14 | −0.08 | −0.01 | |
| 3 M FU | −0.27 ** | −0.21 ** | −0.34 ** | −0.21 ** | −0.19 * | −0.09 | |
| 6 M FU | −0.28 ** | −0.21 ** | −0.37 ** | −0.28 ** | −0.20 ** | 0.02 | |
| 12 M FU | −0.16 * | −0.19 * | −0.23 ** | −0.21 ** | −0.21 ** | 0.00 | |
| 5HETE | Preoperative | 0.11 | 0.00 | 0.11 | 0.03 | 0.04 | −0.01 |
| 1 M FU | 0.06 | −0.16 * | 0.09 | 0.12 | 0.03 | 0.05 | |
| 3 M FU | 0.08 | −0.19 * | 0.03 | 0.04 | 0.11 | −0.02 | |
| 6 M FU | 0.05 | −0.05 | 0.07 | 0.02 | −0.08 | 0.07 | |
| 12 M FU | 0.05 | −0.11 | 0.11 | 0.05 | 0.02 | −0.03 | |
| ΔVascuQol 1 M vs. Preop | ΔVascuQol 3 M vs. Preop | ΔVascuQol 6 M vs. Preop | ΔVascuQol 12 M vs. Preop | ΔVascuQol 8 Y vs. Preop | ||
|---|---|---|---|---|---|---|
| LTE4 | Preoperative | 0.13 | 0.02 | 0.06 | 0.04 | 0.35 ** |
| 1 M FU | −0.21 ** | −0.25 ** | −0.03 | −0.06 | 0.15 | |
| 3 M FU | −0.05 | −0.52 ** | −0.19 * | −0.06 | 0.16 | |
| 6 M FU | 0.02 | −0.24 ** | −0.37 ** | −0.13 | 0.20 * | |
| 12 M FU | 0.06 | −0.15 * | −0.17 * | −0.52 ** | 0.11 | |
| TXB2 | Preoperative | 0.25 ** | 0.07 | 0.09 | 0.09 | 0.35 ** |
| 1 M FU | 0.01 | −0.17 * | 0.02 | 0.06 | 0.17 | |
| 3 M FU | 0.03 | −0.18 * | −0.04 | −0.03 | 0.07 | |
| 6 M FU | 0.04 | −0.20 ** | −0.09 | −0.04 | 0.19 | |
| 12 M FU | −0.03 | −0.12 | −0.10 | −0.11 | 0.10 | |
| 5HETE | Preoperative | −0.08 | 0.04 | −0.04 | −0.03 | −0.10 |
| 1 M FU | −0.18 * | 0.05 | 0.08 | 0.00 | 0.02 | |
| 3 M FU | −0.21 ** | −0.02 | −0.01 | 0.06 | −0.10 | |
| 6 M FU | −0.07 | 0.03 | −0.01 | −0.11 | 0.04 | |
| 12 M FU | −0.13 | 0.07 | 0.02 | −0.02 | −0.07 |
| ΔVascuQol 1 M vs. Preop | ΔVascuQol 3 M vs. Preop | ΔVascuQol 6 M vs. Preop | ΔVascuQol 12 M vs. Preop | ΔVascuQol 8 Y vs. Preop | ||
|---|---|---|---|---|---|---|
| ΔLTE4 | 1 M vs. Preoperative | −0.29 ** | −0.25 ** | −0.07 | −0.08 | −0.11 |
| 3 M vs. Preoperative | −0.14 | −0.53 ** | −0.23 ** | −0.09 | −0.09 | |
| 6 M vs. Preoperative | −0.08 | −0.24 ** | −0.37 ** | −0.15 | −0.08 | |
| 12 M vs. Preoperative | −0.05 | −0.18 * | −0.22 ** | −0.53 ** | −0.17 | |
| ΔTXB2 | 1 M vs. Preoperative | −0.21 ** | −0.24 ** | −0.06 | −0.02 | −0.12 |
| 3 M vs. Preoperative | −0.18 * | −0.23 ** | −0.11 | −0.11 | −0.21 * | |
| 6 M vs. Preoperative | −0.16 * | −0.27 ** | −0.18 * | −0.12 | −0.06 | |
| 12 M vs. Preoperative | −0.21 ** | −0.17 * | −0.17 * | −0.18 * | −0.11 | |
| Δ5HETE | 1 M vs. Preoperative | −0.10 | 0.01 | 0.12 | 0.03 | 0.12 |
| 3 M vs. Preoperative | −0.16 * | −0.06 | 0.03 | 0.09 | −0.01 | |
| 6 M vs. Preoperative | 0.00 | 0.00 | 0.02 | −0.08 | 0.13 | |
| 12 M vs. Preoperative | −0.06 | 0.05 | 0.06 | 0.01 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachsmann-Maga, A.; Maga, M.; Polczyk, R.; Włodarczyk, A.; Pasieka, P.; Terlecki, K.; Maga, P. Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment. J. Clin. Med. 2023, 12, 3412. https://doi.org/10.3390/jcm12103412
Wachsmann-Maga A, Maga M, Polczyk R, Włodarczyk A, Pasieka P, Terlecki K, Maga P. Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment. Journal of Clinical Medicine. 2023; 12(10):3412. https://doi.org/10.3390/jcm12103412
Chicago/Turabian StyleWachsmann-Maga, Agnieszka, Mikołaj Maga, Romuald Polczyk, Aleksandra Włodarczyk, Patrycja Pasieka, Karol Terlecki, and Paweł Maga. 2023. "Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment" Journal of Clinical Medicine 12, no. 10: 3412. https://doi.org/10.3390/jcm12103412
APA StyleWachsmann-Maga, A., Maga, M., Polczyk, R., Włodarczyk, A., Pasieka, P., Terlecki, K., & Maga, P. (2023). Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment. Journal of Clinical Medicine, 12(10), 3412. https://doi.org/10.3390/jcm12103412







