Cells and Materials for Cardiac Repair and Regeneration
Abstract
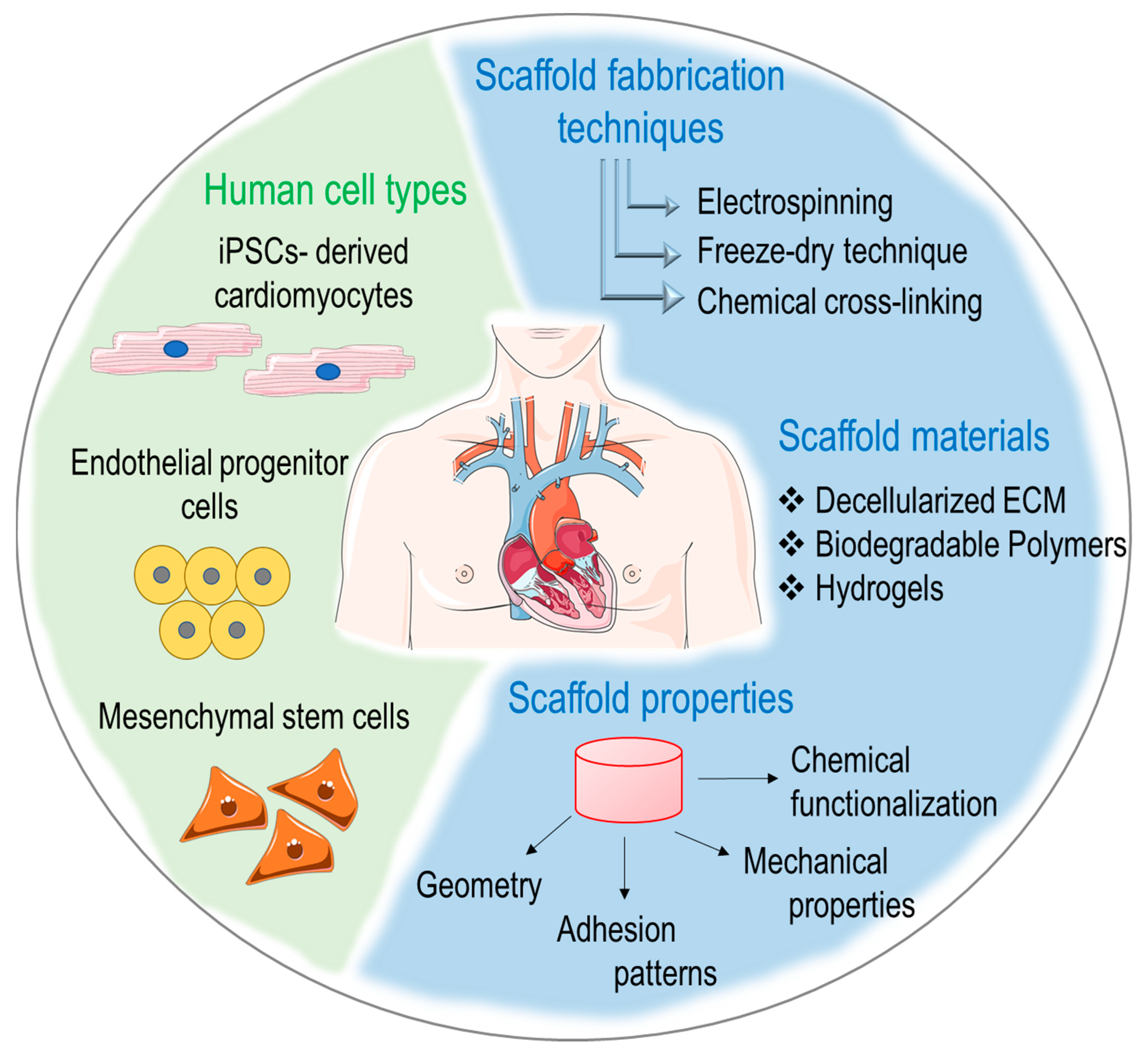
1. Introduction
2. Cell Therapy for Repairing Damaged Myocardium
2.1. Skeletal Myoblasts (SMs)
2.2. Bone Marrow-Derived Cells
2.3. Human Pluripotent Stem Cells
| Cell Type | Advantages | Limitations | Status | Reference |
|---|---|---|---|---|
| Embryonic Stem Cells | High differentiation potential | Possible tumorigenesis | Preclinical Clinical | [76,77,78] |
| Induced Pluripotent Stem Cells | Autologous source, high differentiation potential | Risk of tumorigenesis, insufficient differentiation | Preclinical | [74,79] |
| Skeletal Myoblasts | Contractile properties | Limited engraftment, arrhythmogenic risk | Clinical | [35,36] |
| Cardiosphere-Derived Cells | Pro-angiogenic and immunomodulatory properties, cardiac-specific | Limited engraftment, inconsistent results | Clinical | [80,81] |
| Endothelial Progenitor Cells | Pro-angiogenic properties | Limited differentiation potential and engraftment, inconsistent results | Preclinical Clinical | [82,83] |
| Adipose-Derived Stem Cells | Immunomodulatory and pro-angiogenic properties | Limited differentiation potential and engraftment, inconsistent results | Preclinical Clinical | [84,85] |
3. Tissue Engineering Strategies to Repair/Regenerate the Failing Heart
3.1. Decellularized ECM for Cardiac Engineering
3.2. ECM-Mimicking and -Derived Materials for Cardiac Engineering
3.3. Combining Cells and Biomaterials for Cardiac Tissue Engineering
4. Xenotransplantation and Cardiac Repair
4.1. Immunological Challenges
4.2. Genetic Engineering Strategies of Animals and Cells to Ensure Immunological Compatibility
5. Harnessing Cell Mechano-Sensation to Repair/Regenerate the Heart
5.1. From Force Decryption to Intracellular Signaling
5.2. Mechanotransduction in Heart Physiology and Pathology
5.3. Mechanical-Dependent Pathologic Signaling
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemmo, S.I.; Naser, A.Y.; Alwafi, H.; Mansour, M.M.; Alanazi, A.F.R.; Jalal, Z.; Alsairafi, Z.K.; Paudyal, V.; Alomari, E.; Al-Momani, H.; et al. Hospital Admissions Due to Ischemic Heart Diseases and Prescriptions of Cardiovascular Diseases Medications in England and Wales in the Past Two Decades. Int. J. Environ. Res. Public Health 2021, 18, 7041. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, N.; Francis, J.; Fihn, S.D.; Meyer, C.S.; Whooley, M.A. Leading causes of cardiovascular hospitalization in 8.45 million US veterans. PLoS ONE 2018, 13, e0193996. [Google Scholar] [CrossRef]
- Beltran-Camacho, L.; Rojas-Torres, M.; Duran-Ruiz, M.C. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int. J. Mol. Sci. 2021, 22, 2335. [Google Scholar] [CrossRef] [PubMed]
- Desgres, M.; Menasche, P. Clinical Translation of Pluripotent Stem Cell Therapies: Challenges and Considerations. Cell Stem Cell 2019, 25, 594–606. [Google Scholar] [CrossRef]
- Martinez-Falguera, D.; Iborra-Egea, O.; Galvez-Monton, C. iPSC Therapy for Myocardial Infarction in Large Animal Models: Land of Hope and Dreams. Biomedicines 2021, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Soma, Y.; Morita, Y.; Kishino, Y.; Kanazawa, H.; Fukuda, K.; Tohyama, S. The Present State and Future Perspectives of Cardiac Regenerative Therapy Using Human Pluripotent Stem Cells. Front. Cardiovasc. Med. 2021, 8, 774389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, Y.; Pan, T.; Zhu, X.; Chong, H.; Xu, C.; Fan, F.; Cao, H.; Zhang, B.; Pan, J.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Bolli, R. A realistic appraisal of the use of embryonic stem cell-based therapies for cardiac repair. Eur. Heart J. 2020, 41, 2397–2404. [Google Scholar] [CrossRef]
- Katz, A.M.; Rolett, E.L. Heart failure: When form fails to follow function. Eur. Heart J. 2016, 37, 449–454. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Buren, P.V.; Meyer, M.; et al. Myocardial Stiffness in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- VanDusen, N.J.; Lee, J.Y.; Gu, W.; Butler, C.E.; Sethi, I.; Zheng, Y.; King, J.S.; Zhou, P.; Suo, S.; Guo, Y.; et al. Massively parallel in vivo CRISPR screening identifies RNF20/40 as epigenetic regulators of cardiomyocyte maturation. Nat. Commun. 2021, 12, 4442. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Santoro, R. Feeling the right force: How to contextualize the cell mechanical behavior in physiologic turnover and pathologic evolution of the cardiovascular system. Pharmacol. Ther. 2017, 171, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, H.; Rah, W.; Chang, H.J.; Yoon, Y.S. From engineered heart tissue to cardiac organoid. Theranostics 2022, 12, 2758–2772. [Google Scholar] [CrossRef]
- Pesce, M.; Burba, I.; Gambini, E.; Prandi, F.; Pompilio, G.; Capogrossi, M.C. Endothelial and cardiac progenitors: Boosting, conditioning and (re)programming for cardiovascular repair. Pharmacol Ther. 2011, 129, 50–61. [Google Scholar] [CrossRef]
- Tucker, N.R.; Chaffin, M.; Fleming, S.J.; Hall, A.W.; Parsons, V.A.; Bedi, K.C., Jr.; Akkad, A.D.; Herndon, C.N.; Arduini, A.; Papangeli, I.; et al. Transcriptional and Cellular Diversity of the Human Heart. Circulation 2020, 142, 466–482. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Romero, J.P.; Hernandez, S.C.; Vilas-Zornoza, A.; Fortelny, N.; Castro-Labrador, L.; San Martin-Uriz, P.; Lorenzo-Vivas, E.; Garcia-Olloqui, P.; Palacio, M.; et al. Single-Cell RNA Sequencing Analysis Reveals a Crucial Role for CTHRC1 (Collagen Triple Helix Repeat Containing 1) Cardiac Fibroblasts After Myocardial Infarction. Circulation 2020, 142, 1831–1847. [Google Scholar] [CrossRef]
- Hesse, J.; Owenier, C.; Lautwein, T.; Zalfen, R.; Weber, J.F.; Ding, Z.; Alter, C.; Lang, A.; Grandoch, M.; Gerdes, N.; et al. Single-cell transcriptomics defines heterogeneity of epicardial cells and fibroblasts within the infarcted murine heart. eLife 2021, 10, e65921. [Google Scholar] [CrossRef]
- Nicin, L.; Wagner, J.U.G.; Luxan, G.; Dimmeler, S. Fibroblast-mediated intercellular crosstalk in the healthy and diseased heart. FEBS Lett. 2022, 596, 638–654. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.R.; Henrikson, C.A.; Tung, L.; Chang, M.G.; Aon, M.; Xue, T.; Li, R.A.; Rourke, B.O.; Marban, E. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ. Res. 2005, 97, 159–167. [Google Scholar] [CrossRef]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef]
- Formigli, L.; Zecchi-Orlandini, S.; Meacci, E.; Bani, D. Skeletal myoblasts for heart regeneration and repair: State of the art and perspectives on the mechanisms for functional cardiac benefits. Curr. Pharm. Des. 2010, 16, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Al Attar, N.; Carrion, C.; Ghostine, S.; Garcin, I.; Vilquin, J.T.; Hagege, A.A.; Menasche, P. Long-term (1 year) functional and histological results of autologous skeletal muscle cells transplantation in rat. Cardiovasc. Res. 2003, 58, 142–148. [Google Scholar] [CrossRef]
- Suzuki, K.; Murtuza, B.; Suzuki, N.; Smolenski, R.T.; Yacoub, M.H. Intracoronary infusion of skeletal myoblasts improves cardiac function in doxorubicin-induced heart failure. Circulation 2001, 104, I213–I217. [Google Scholar] [CrossRef]
- Bonaros, N.; Rauf, R.; Wolf, D.; Margreiter, E.; Tzankov, A.; Schlechta, B.; Kocher, A.; Ott, H.; Schachner, T.; Hering, S.; et al. Combined transplantation of skeletal myoblasts and angiopoietic progenitor cells reduces infarct size and apoptosis and improves cardiac function in chronic ischemic heart failure. J. Thorac. Cardiovasc. Surg. 2006, 132, 1321–1328. [Google Scholar] [CrossRef]
- Farahmand, P.; Lai, T.Y.; Weisel, R.D.; Fazel, S.; Yau, T.; Menasche, P.; Li, R.K. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation 2008, 118, S130–S137. [Google Scholar] [CrossRef]
- Menasche, P.; Hagege, A.A.; Vilquin, J.T.; Desnos, M.; Abergel, E.; Pouzet, B.; Bel, A.; Sarateanu, S.; Scorsin, M.; Schwartz, K.; et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 1078–1083. [Google Scholar] [CrossRef]
- Smits, P.C.; van Geuns, R.J.; Poldermans, D.; Bountioukos, M.; Onderwater, E.E.; Lee, C.H.; Maat, A.P.; Serruys, P.W. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. J. Am. Coll. Cardiol. 2003, 42, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Herreros, J.; Prosper, F.; Perez, A.; Gavira, J.J.; Garcia-Velloso, M.J.; Barba, J.; Sanchez, P.L.; Canizo, C.; Rabago, G.; Marti-Climent, J.M.; et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur. Heart J. 2003, 24, 2012–2020. [Google Scholar] [CrossRef]
- Siminiak, T.; Kalawski, R.; Fiszer, D.; Jerzykowska, O.; Rzezniczak, J.; Rozwadowska, N.; Kurpisz, M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up. Am. Heart J. 2004, 148, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Dib, N.; Michler, R.E.; Pagani, F.D.; Wright, S.; Kereiakes, D.J.; Lengerich, R.; Binkley, P.; Buchele, D.; Anand, I.; Swingen, C.; et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: Four-year follow-up. Circulation 2005, 112, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.J.; Herreros, J.; Perez, A.; Garcia-Velloso, M.J.; Barba, J.; Martin-Herrero, F.; Canizo, C.; Martin-Arnau, A.; Marti-Climent, J.M.; Hernandez, M.; et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J. Thorac. Cardiovasc. Surg. 2006, 131, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Biagini, E.; Valgimigli, M.; Smits, P.C.; Poldermans, D.; Schinkel, A.F.; Rizzello, V.; Onderwater, E.E.; Bountioukos, M.; Serruys, P.W. Stress and tissue Doppler echocardiographic evidence of effectiveness of myoblast transplantation in patients with ischaemic heart failure. Eur. J. Heart Fail. 2006, 8, 641–648. [Google Scholar] [CrossRef]
- Siminiak, T.; Fiszer, D.; Jerzykowska, O.; Grygielska, B.; Rozwadowska, N.; Kalmucki, P.; Kurpisz, M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: The POZNAN trial. Eur. Heart J. 2005, 26, 1188–1195. [Google Scholar] [CrossRef]
- Ince, H.; Petzsch, M.; Rehders, T.C.; Chatterjee, T.; Nienaber, C.A. Transcatheter transplantation of autologous skeletal myoblasts in postinfarction patients with severe left ventricular dysfunction. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2004, 11, 695–704. [Google Scholar] [CrossRef]
- Reinecke, H.; Poppa, V.; Murry, C.E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J. Mol. Cell. Cardiol. 2002, 34, 241–249. [Google Scholar] [CrossRef]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef]
- De Falco, E.; Porcelli, D.; Torella, A.R.; Straino, S.; Iachininoto, M.G.; Orlandi, A.; Truffa, S.; Biglioli, P.; Napolitano, M.; Capogrossi, M.C.; et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 2004, 104, 3472–3482. [Google Scholar] [CrossRef]
- Bel, A.; Messas, E.; Agbulut, O.; Richard, P.; Samuel, J.L.; Bruneval, P.; Hagege, A.A.; Menasche, P. Transplantation of autologous fresh bone marrow into infarcted myocardium: A word of caution. Circulation 2003, 108 (Suppl. S1), II-247–II-252. [Google Scholar] [CrossRef]
- Moelker, A.D.; Baks, T.; van den Bos, E.J.; van Geuns, R.J.; de Feyter, P.J.; Duncker, D.J.; van der Giessen, W.J. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur. Heart J. 2006, 27, 3057–3064. [Google Scholar] [CrossRef]
- Yokoyama, S.; Fukuda, N.; Li, Y.; Hagikura, K.; Takayama, T.; Kunimoto, S.; Honye, J.; Saito, S.; Wada, M.; Satomi, A.; et al. A strategy of retrograde injection of bone marrow mononuclear cells into the myocardium for the treatment of ischemic heart disease. J. Mol. Cell. Cardiol. 2006, 40, 24–34. [Google Scholar] [CrossRef]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef]
- Mathieu, M.; Bartunek, J.; El Oumeiri, B.; Touihri, K.; Hadad, I.; Thoma, P.; Metens, T.; da Costa, A.M.; Mahmoudabady, M.; Egrise, D.; et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J. Thorac. Cardiovasc. Surg. 2009, 138, 646–653. [Google Scholar] [CrossRef]
- Vinci, M.C.; Polvani, G.; Pesce, M. Epigenetic programming and risk: The birthplace of cardiovascular disease? Stem Cell. Rev. Rep. 2013, 9, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Lehmann, R.; Honold, J.; Assmus, B.; Aicher, A.; Walter, D.H.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004, 109, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, V.V.; Krylov, A.L.; Poponina, Y.S.; Maslov, L.N. Cardiac contractility after transplantation of autologous mononuclear bone marrow cells in patients with myocardial infarction. Bull. Exp. Biol. Med. 2006, 141, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Stamm, C.; Westphal, B.; Kleine, H.D.; Petzsch, M.; Kittner, C.; Klinge, H.; Schumichen, C.; Nienaber, C.A.; Freund, M.; Steinhoff, G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003, 361, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, W.; Tendera, M.; Zebzda, A.; Michalowska, A.; Majka, M.; Kucia, M.; Maslankiewicz, K.; Wyderka, R.; Krol, M.; Ochala, A.; et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur. Heart J. 2006, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Aceves, J.L.; Lopez, R.V.; Teran, P.M.; Escobedo, C.M.; Marroquin Mucino, M.A.; Castillo, G.G.; Estrada, M.M.; Garcia, F.R.; Quiroz, G.D.; Montano Estrada, L.F. Autologous CXCR4+ Hematopoietic Stem Cells Injected into the Scar Tissue of Chronic Myocardial Infarction Patients Normalizes Tissue Contractility and Perfusion. Arch. Med. Res. 2020, 51, 135–144. [Google Scholar] [CrossRef]
- Rai, B.; Shukla, J.; Henry, T.D.; Quesada, O. Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair-A Systematic Review. Cells 2021, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Quevedo, P.; Gonzalez-Ferrer, J.J.; Sabate, M.; Garcia-Moll, X.; Delgado-Bolton, R.; Llorente, L.; Bernardo, E.; Ortega-Pozzi, A.; Hernandez-Antolin, R.; Alfonso, F.; et al. Selected CD133(+) progenitor cells to promote angiogenesis in patients with refractory angina: Final results of the PROGENITOR randomized trial. Circ. Res. 2014, 115, 950–960. [Google Scholar] [CrossRef]
- Perrotta, F.; Perna, A.; Komici, K.; Nigro, E.; Mollica, M.; D’Agnano, V.; De Luca, A.; Guerra, G. The State of Art of Regenerative Therapy in Cardiovascular Ischemic Disease: Biology, Signaling Pathways, and Epigenetics of Endothelial Progenitor Cells. Cells 2020, 9, 1886. [Google Scholar] [CrossRef]
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: Key players towards the definition of vascular competence. J. Cell. Mol. Med. 2009, 13, 454–471. [Google Scholar] [CrossRef]
- Zhang, Y.; Sivakumaran, P.; Newcomb, A.E.; Hernandez, D.; Harris, N.; Khanabdali, R.; Liu, G.S.; Kelly, D.J.; Pebay, A.; Hewitt, A.W.; et al. Cardiac Repair With a Novel Population of Mesenchymal Stem Cells Resident in the Human Heart. Stem Cells 2015, 33, 3100–3113. [Google Scholar] [CrossRef]
- Ward, M.R.; Abadeh, A.; Connelly, K.A. Concise Review: Rational Use of Mesenchymal Stem Cells in the Treatment of Ischemic Heart Disease. Stem Cells Transl. Med. 2018, 7, 543–550. [Google Scholar] [CrossRef]
- Schuleri, K.H.; Feigenbaum, G.S.; Centola, M.; Weiss, E.S.; Zimmet, J.M.; Turney, J.; Kellner, J.; Zviman, M.M.; Hatzistergos, K.E.; Detrick, B.; et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart J. 2009, 30, 2722–2732. [Google Scholar] [CrossRef]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Suncion, V.Y.; McCall, F.; Guerra, D.; Mather, J.; Zambrano, J.P.; Heldman, A.W.; Hare, J.M. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J. Am. Heart Assoc. 2013, 2, e000140. [Google Scholar] [CrossRef]
- Gubert, F.; da Silva, J.S.; Vasques, J.F.; de Jesus Goncalves, R.G.; Martins, R.S.; de Sa, M.P.L.; Mendez-Otero, R.; Zapata-Sudo, G. Mesenchymal Stem Cells Therapies on Fibrotic Heart Diseases. Int. J. Mol. Sci. 2021, 22, 7447. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Graichen, R.; Xu, X.; Braam, S.R.; Balakrishnan, T.; Norfiza, S.; Sieh, S.; Soo, S.Y.; Tham, S.C.; Mummery, C.; Colman, A.; et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation 2008, 76, 357–370. [Google Scholar] [CrossRef]
- Xu, X.Q.; Graichen, R.; Soo, S.Y.; Balakrishnan, T.; Rahmat, S.N.; Sieh, S.; Tham, S.C.; Freund, C.; Moore, J.; Mummery, C.; et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 2008, 76, 958–970. [Google Scholar] [CrossRef]
- Kehat, I.; Khimovich, L.; Caspi, O.; Gepstein, A.; Shofti, R.; Arbel, G.; Huber, I.; Satin, J.; Itskovitz-Eldor, J.; Gepstein, L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004, 22, 1282–1289. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, Y.; Liu, H.; Tang, L.; Du, R.; Shen, Y.; Feng, J.; Zhang, K.; Xu, C.; Zhang, S.; et al. In Vivo Dynamic Metabolic Changes After Transplantation of Induced Pluripotent Stem Cells for Ischemic Injury. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 2012–2015. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous Induced Pluripotent Stem Cell-Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388. [Google Scholar] [CrossRef] [PubMed]
- Blin, G.; Nury, D.; Stefanovic, S.; Neri, T.; Guillevic, O.; Brinon, B.; Bellamy, V.; Rucker-Martin, C.; Barbry, P.; Bel, A.; et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Investig. 2010, 120, 1125–1139. [Google Scholar] [CrossRef]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef]
- Ménard, C.; Hagège, A.A.; Agbulut, O.; Barro, M.; Morichetti, M.C.; Brasselet, C.; Bel, A.; Messas, E.; Bissery, A.; Bruneval, P.; et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet 2005, 366, 1005–1012. [Google Scholar] [CrossRef]
- Masumoto, H.; Nakane, T.; Tinney, J.P.; Yuan, F.; Ye, F.; Kowalski, W.J.; Minakata, K.; Sakata, R.; Yamashita, J.K.; Keller, B.B. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci. Rep. 2016, 6, 29933. [Google Scholar] [CrossRef]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.; Liehn, E.A.; Sasse, A.; Hristov, M.; Sobota, R.; Kelm, M.; Merx, M.W.; Weber, C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic. Res. Cardiol. 2008, 103, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ramírez, R.; Uribe-Longoria, A.; Rangel-Fuentes, M.M.; Gutiérrez-Fajardo, P.; Salazar-Riojas, R.; Cervantes-García, D.; Treviño-Ortiz, J.H.; Benavides-Chereti, G.J.; Espinosa-Oliveros, L.P.; Limón-Rodríguez, R.H.; et al. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc. Revasc Med. 2010, 11, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Johnstone, B.H.; Cook, T.G.; Tan, J.; Fishbein, M.C.; Chen, P.S.; March, K.L. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 2009, 27, 230–237. [Google Scholar] [CrossRef]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95.e82. [Google Scholar] [CrossRef]
- Miyamoto, M.; Nam, L.; Kannan, S.; Kwon, C. Heart organoids and tissue models for modeling development and disease. Semin. Cell. Dev. Biol. 2021, 118, 119–128. [Google Scholar] [CrossRef]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Garoffolo, G.; Ferrari, S.; Rizzi, S.; Barbuto, M.; Bernava, G.; Pesce, M. Harnessing Mechanosensation in Next Generation Cardiovascular Tissue Engineering. Biomolecules 2020, 10, 1419. [Google Scholar] [CrossRef]
- Rizzi, S.; Mantero, S.; Boschetti, F.; Pesce, M. Luminal endothelialization of small caliber silk tubular graft for vascular constructs engineering. Front. Cardiovasc. Med. 2022, 9, 1013183. [Google Scholar] [CrossRef]
- Scafa Udriste, A.; Niculescu, A.G.; Iliuta, L.; Bajeu, T.; Georgescu, A.; Grumezescu, A.M.; Badila, E. Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers 2023, 15, 1177. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.; Ragazzini, S.; Pesce, M. Engineering Efforts to Refine Compatibility and Duration of Aortic Valve Replacements: An Overview of Previous Expectations and New Promises. Front. Cardiovasc. Med. 2022, 9, 863136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Frazier, O.H.; Elgalad, A.; Hochman-Mendez, C.; Sampaio, L.C. Building a Total Bioartificial Heart: Harnessing Nature to Overcome the Current Hurdles. Artif. Organs 2018, 42, 970–982. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, e1801217. [Google Scholar] [CrossRef]
- Krishnan, A.; Wang, H.; MacArthur, J.W. Applications of Tissue Decellularization Techniques in Ventricular Myocardial Biofabrication. Front. Bioeng. Biotechnol. 2022, 10, 802283. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Sobhani, S.; Soltani Khaboushan, A.; Kajbafzadeh, A.M. Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises. Bioengineering 2023, 10, 106. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Uriarte, J.J.; Prat-Vidal, C.; Galvez-Monton, C.; Roura, S.; Llucia-Valldeperas, A.; Soler-Botija, C.; Farre, R.; Navajas, D.; Bayes-Genis, A. In vitro comparative study of two decellularization protocols in search of an optimal myocardial scaffold for recellularization. Am. J. Transl. Res. 2015, 7, 558–573. [Google Scholar]
- Vinci, M.C.; Tessitore, G.; Castiglioni, L.; Prandi, F.; Soncini, M.; Santoro, R.; Consolo, F.; Colazzo, F.; Micheli, B.; Sironi, L.; et al. Mechanical compliance and immunological compatibility of fixative-free decellularized/cryopreserved human pericardium. PLoS ONE 2013, 8, e64769. [Google Scholar] [CrossRef]
- Akhyari, P.; Aubin, H.; Gwanmesia, P.; Barth, M.; Hoffmann, S.; Huelsmann, J.; Preuss, K.; Lichtenberg, A. The quest for an optimized protocol for whole-heart decellularization: A comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng. Part C Methods 2011, 17, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Consolo, F.; Spiccia, M.; Piola, M.; Kassem, S.; Prandi, F.; Vinci, M.C.; Forti, E.; Polvani, G.; Fiore, G.B.; et al. Feasibility of pig and human-derived aortic valve interstitial cells seeding on fixative-free decellularized animal pericardium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 345–356. [Google Scholar] [CrossRef]
- Amadeo, F.; Barbuto, M.; Bernava, G.; Savini, N.; Brioschi, M.; Rizzi, S.; Banfi, C.; Polvani, G.; Pesce, M. Culture Into Perfusion-Assisted Bioreactor Promotes Valve-Like Tissue Maturation of Recellularized Pericardial Membrane. Front. Cardiovasc. Med. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, F.; Boschetti, F.; Polvani, G.; Banfi, C.; Pesce, M.; Santoro, R. Aortic valve cell seeding into decellularized animal pericardium by perfusion-assisted bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Methe, K.; Bäckdahl, H.; Johansson, B.R.; Nayakawde, N.; Dellgren, G.; Sumitran-Holgersson, S. An alternative approach to decellularize whole porcine heart. Biores. Open Access 2014, 3, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.Y.; Sklaviadis, D.; Tochka, Z.L.; Fischer, K.M.; Hearon, K.; Morgan, T.D.; Langer, R.; Freed, L.E. Multi-Material Tissue Engineering Scaffold with Hierarchical Pore Architecture. Adv. Funct. Mater. 2016, 26, 5873–5883. [Google Scholar] [CrossRef]
- Sugiura, T.; Matsumura, G.; Miyamoto, S.; Miyachi, H.; Breuer, C.K.; Shinoka, T. Tissue-engineered Vascular Grafts in Children With Congenital Heart Disease: Intermediate Term Follow-up. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 175–179. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Wang, Z.; Wen, D.; Zhang, W.; Schmull, S.; Li, H.; Chen, Y.; Xue, S. Electrospun nanofibrous sheets of collagen/elastin/polycaprolactone improve cardiac repair after myocardial infarction. Am. J. Transl. Res. 2016, 8, 1678–1694. [Google Scholar]
- Wee, J.H.; Yoo, K.D.; Sim, S.B.; Kim, H.J.; Kim, H.J.; Park, K.N.; Kim, G.H.; Moon, M.H.; You, S.J.; Ha, M.Y.; et al. Stem cell laden nano and micro collagen/PLGA bimodal fibrous patches for myocardial regeneration. Biomater. Res. 2022, 26, 79. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef]
- Sridharan, D.; Palaniappan, A.; Blackstone, B.N.; Powell, H.M.; Khan, M. Electrospun Aligned Coaxial Nanofibrous Scaffold for Cardiac Repair. Methods Mol. Biol. 2021, 2193, 129–140. [Google Scholar] [CrossRef]
- Lv, J.; Liu, W.; Shi, G.; Zhu, F.; He, X.; Zhu, Z.; Chen, H. Human cardiac extracellular matrix-chitosan-gelatin composite scaffold and its endothelialization. Exp. Ther. Med. 2020, 19, 1225–1234. [Google Scholar] [CrossRef]
- Hochman-Mendez, C.; Pereira de Campos, D.B.; Pinto, R.S.; Mendes, B.; Rocha, G.M.; Monnerat, G.; Weissmuller, G.; Sampaio, L.C.; Carvalho, A.B.; Taylor, D.A.; et al. Tissue-engineered human embryonic stem cell-containing cardiac patches: Evaluating recellularization of decellularized matrix. J. Tissue Eng. 2020, 11, 2041731420921482. [Google Scholar] [CrossRef]
- Mesquita, F.C.P.; Morrissey, J.; Lee, P.F.; Monnerat, G.; Xi, Y.; Andersson, H.; Nogueira, F.C.S.; Domont, G.B.; Sampaio, L.C.; Hochman-Mendez, C.; et al. Cues from human atrial extracellular matrix enrich the atrial differentiation of human induced pluripotent stem cell-derived cardiomyocytes. Biomater. Sci. 2021, 9, 3737–3749. [Google Scholar] [CrossRef]
- Al-Hejailan, R.S.; Bakheet, R.H.; Al-Saud, M.M.; Al-Jufan, M.B.; Al-Hindas, H.M.; Al-Qattan, S.M.; Al-Muhanna, M.K.; Parhar, R.S.; Conca, W.; Hansmann, J.; et al. Toward allogenizing a xenograft: Xenogeneic cardiac scaffolds recellularized with human-induced pluripotent stem cells do not activate human naive neutrophils. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 691–701. [Google Scholar] [CrossRef]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef]
- Amin, D.R.; Sink, E.; Narayan, S.P.; Abdel-Hafiz, M.; Mestroni, L.; Pena, B. Nanomaterials for Cardiac Tissue Engineering. Molecules 2020, 25, 5189. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, D.E.; Kobashigawa, J.A. Heart Transplantation for Advanced Heart Failure. Card. Intensive Care 2019, 504–524.e502. [Google Scholar] [CrossRef]
- Cooper, D.K. A brief history of cross-species organ transplantation. Proc. Bayl. Univ. Med. Cent. 2012, 25, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Li, P.; Cooper, D.K.C. Xenotransplantation: Past, present, and future. Curr. Opin. Organ. Transpl. 2017, 22, 513–521. [Google Scholar] [CrossRef]
- Garry, D.J.; Weiner, J.I.; Greising, S.M.; Garry, M.G.; Sachs, D.H. Mechanisms and strategies to promote cardiac xenotransplantation. J. Mol. Cell. Cardiol. 2022, 172, 109–119. [Google Scholar] [CrossRef]
- Hawthorne, W.J. World first pig-to-human cardiac xenotransplantation. Xenotransplantation 2022, 29, e12733. [Google Scholar] [CrossRef]
- Galili, U. Evolution of α1,3Galactosyltransferase and of the α-Gal Epitope. In α-Gal and Anti-Gal: α1,3-Galactosyltransferase, α-Gal Epitopes, and the Natural Anti-Gal Antibody Subcellular Biochemistry; Galili, U., Avila, J.L., Eds.; Springer: Boston, MA, USA, 1999; pp. 1–23. [Google Scholar] [CrossRef]
- Galili, U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol. Today 1993, 14, 480–482. [Google Scholar] [CrossRef]
- Kobayashi, T.; Cooper, D.K. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell. Biochem. 1999, 32, 229–257. [Google Scholar] [CrossRef]
- Houser, S.L.; Kuwaki, K.; Knosalla, C.; Dor, F.J.; Gollackner, B.; Cheng, J.; Shimizu, A.; Schuurman, H.J.; Cooper, D.K. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 2004, 11, 416–425. [Google Scholar] [CrossRef]
- Buhler, L.; Basker, M.; Alwayn, I.P.; Goepfert, C.; Kitamura, H.; Kawai, T.; Gojo, S.; Kozlowski, T.; Ierino, F.L.; Awwad, M.; et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 2000, 70, 1323–1331. [Google Scholar] [CrossRef]
- Yamada, K.; Sachs, D.H.; DerSimonian, H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J. Immunol. 1995, 155, 5249–5256. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bolling, S.F.; Linsley, P.S.; Wei, R.Q.; Gordon, D.; Thompson, C.B.; Turka, L.A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J. Exp. Med. 1993, 178, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Elwood, E.T.; Larsen, C.P.; Cho, H.R.; Corbascio, M.; Ritchie, S.C.; Alexander, D.Z.; Tucker-Burden, C.; Linsley, P.S.; Aruffo, A.; Hollenbaugh, D.; et al. Prolonged acceptance of concordant and discordant xenografts with combined cd40 and cd28 pathway blockade1. Transplantation 1998, 65, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, A.M.; Mottram, P.L.; Han, W.; Walters, S.N.; Patel, A.T.; Hawthorne, W.J.; Cowan, P.J.; d’Apice, A.J.; O’Connell, P.J. Blockade of the CD28 and CD40 pathways result in the acceptance of pig and rat islet xenografts but not rat cardiac grafts in mice. Transpl. Immunol. 2001, 9, 51–56. [Google Scholar] [CrossRef]
- Wu, G.; Pfeiffer, S.; Schroder, C.; Zhang, T.; Nguyen, B.N.; Lea, W.; Kelishadi, S.; Atkinson, J.B.; Schuurman, H.J.; White, D.J.; et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 2005, 12, 197–208. [Google Scholar] [CrossRef]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef]
- Kolber-Simonds, D.; Lai, L.; Watt, S.R.; Denaro, M.; Arn, S.; Augenstein, M.L.; Betthauser, J.; Carter, D.B.; Greenstein, J.L.; Hao, Y.; et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 7335–7340. [Google Scholar] [CrossRef]
- Ezzelarab, M.; Garcia, B.; Azimzadeh, A.; Sun, H.; Lin, C.C.; Hara, H.; Kelishadi, S.; Zhang, T.; Lin, Y.J.; Tai, H.C.; et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation 2009, 87, 805–812. [Google Scholar] [CrossRef]
- Hara, H.; Long, C.; Lin, Y.J.; Tai, H.C.; Ezzelarab, M.; Ayares, D.; Cooper, D.K. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl. Int. 2008, 21, 1163–1174. [Google Scholar] [CrossRef]
- McGregor, C.G.; Ricci, D.; Miyagi, N.; Stalboerger, P.G.; Du, Z.; Oehler, E.A.; Tazelaar, H.D.; Byrne, G.W. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation 2012, 93, 686–692. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Varki, A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.J.; Li, P.; Estrada, J.L.; Sidner, R.A.; Chihara, R.K.; Downey, S.M.; Burlak, C.; Wang, Z.Y.; Reyes, L.M.; Ivary, B.; et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Ball, S.F.; Vaught, T.D.; Vance, A.M.; Mendicino, M.; Monahan, J.A.; Walters, A.H.; Wells, K.D.; Dandro, A.S.; Ramsoondar, J.J.; et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 2009, 16, 477–485. [Google Scholar] [CrossRef]
- Plege, A.; Borns, K.; Baars, W.; Schwinzer, R. Suppression of human T-cell activation and expansion of regulatory T cells by pig cells overexpressing PD-ligands. Transplantation 2009, 87, 975–982. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Ezzelarab, M.; Iwase, H.; Hara, H. Perspectives on the Optimal Genetically Engineered Pig in 2018 for Initial Clinical Trials of Kidney or Heart Xenotransplantation. Transplantation 2018, 102, 1974–1982. [Google Scholar] [CrossRef]
- Garoffolo, G.; Pesce, M. Mechanotransduction in the Cardiovascular System: From Developmental Origins to Homeostasis and Pathology. Cells 2019, 8, 1607. [Google Scholar] [CrossRef]
- Garoffolo, G.; Madonna, R.; de Caterina, R.; Pesce, M. Cell based mechanosensing in vascular patho-biology: More than a simple go-with the flow. Vasc. Pharm. 2018, 111, 7–14. [Google Scholar] [CrossRef]
- Roca-Cusachs, P.; del Rio, A.; Puklin-Faucher, E.; Gauthier, N.C.; Biais, N.; Sheetz, M.P. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. USA 2013, 110, E1361–E1370. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Chen, L.; Chan, S.W.; Zhang, X.; Walsh, M.; Lim, C.J.; Hong, W.; Song, H. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes. Dev. 2010, 24, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Iyer, K.V.; Kumar, A.; Shivashankar, G.V. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11349–11354. [Google Scholar] [CrossRef] [PubMed]
- Garoffolo, G.; Casaburo, M.; Amadeo, F.; Salvi, M.; Bernava, G.; Piacentini, L.; Chimenti, I.; Zaccagnini, G.; Milcovich, G.; Zuccolo, E.; et al. Reduction of Cardiac Fibrosis by Interference With YAP-Dependent Transactivation. Circ. Res. 2022, 131, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Laschinger, C.; Arora, P.; Szaszi, K.; Kapus, A.; McCulloch, C.A. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell. Sci. 2007, 120, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; Thatcher, J.E.; Sutherland, L.B.; Kinoshita, H.; Gerard, R.D.; Richardson, J.A.; Dimaio, J.M.; Sadek, H.; Kuwahara, K.; Olson, E.N. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 2010, 107, 294–304. [Google Scholar] [CrossRef]
- Happe, C.L.; Engler, A.J. Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis. Circ. Res. 2016, 118, 296–310. [Google Scholar] [CrossRef]
- Chiou, K.K.; Rocks, J.W.; Chen, C.Y.; Cho, S.; Merkus, K.E.; Rajaratnam, A.; Robison, P.; Tewari, M.; Vogel, K.; Majkut, S.F.; et al. Mechanical signaling coordinates the embryonic heartbeat. Proc. Natl. Acad. Sci. USA 2016, 113, 8939–8944. [Google Scholar] [CrossRef]
- Majkut, S.; Idema, T.; Swift, J.; Krieger, C.; Liu, A.; Discher, D.E. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol. CB 2013, 23, 2434–2439. [Google Scholar] [CrossRef]
- Majkut, S.; Dingal, P.C.; Discher, D.E. Stress sensitivity and mechanotransduction during heart development. Curr. Biol. CB 2014, 24, R495–R501. [Google Scholar] [CrossRef]
- Notari, M.; Ventura-Rubio, A.; Bedford-Guaus, S.J.; Jorba, I.; Mulero, L.; Navajas, D.; Marti, M.; Raya, A. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 2018, 4, eaao5553. [Google Scholar] [CrossRef]
- Yu, J.K.; Sarathchandra, P.; Chester, A.; Yacoub, M.; Brand, T.; Butcher, J.T. Cardiac regeneration following cryoinjury in the adult zebrafish targets a maturation-specific biomechanical remodeling program. Sci. Rep. 2018, 8, 15661. [Google Scholar] [CrossRef] [PubMed]
- Yahalom-Ronen, Y.; Rajchman, D.; Sarig, R.; Geiger, B.; Tzahor, E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife 2015, 4, e07455. [Google Scholar] [CrossRef] [PubMed]
- Canseco, D.C.; Kimura, W.; Garg, S.; Mukherjee, S.; Bhattacharya, S.; Abdisalaam, S.; Das, S.; Asaithamby, A.; Mammen, P.P.; Sadek, H.A. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 2015, 65, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Duda, G.N.; Forte, G.; Girao, H.; Raya, A.; Roca-Cusachs, P.; Sluijter, J.P.G.; Tschope, C.; Van Linthout, S. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nat. Rev. Cardiol. 2023, 20, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Garoffolo, G.; Pesce, M. From dissection of fibrotic pathways to assessment of drug interactions to reduce cardiac fibrosis and heart failure. Curr. Res. Pharm. Drug. Discov. 2021, 2, 100036. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef]
- Ragazzini, S.; Scocozza, F.; Bernava, G.; Auricchio, F.; Colombo, G.I.; Barbuto, M.; Conti, M.; Pesce, M.; Garoffolo, G. Mechanosensor YAP cooperates with TGF-beta1 signaling to promote myofibroblast activation and matrix stiffening in a 3D model of human cardiac fibrosis. Acta Biomater. 2022, 152, 300–312. [Google Scholar] [CrossRef]
| Scaffold | Technique | Results | Year | Reference |
|---|---|---|---|---|
| Conductive nanofiber scaffold (polypyrrole hydrogel/chitosan/polyethylene oxide) | Electrospinning | Cell adhesion, growth and proliferation, conductive nanofiber scaffolds appropriate to use in cardiac tissue engineering. | 2021 | [112] |
| Polypyrrole scaffold coated with silk fibroin | Electrospinning | Mimic of myocardium fibrils, resemble mechanical properties to the native myocardium, good electrical conductivity for cardiomyocytes, and support CM contraction. | 2021 | [106] |
| Composite of cardiac ECM with alginate and chitosan | Freeze-dry technique | Very high swelling rate and porosity, stability in PBS solution, improving of the tensile strength, proliferation of human MSC inside the pores, high marker cTnT expression | 2020 | [113] |
| (Collagen/carbon nano tubes/chitosan/gold nanoparticles) Injectable hydrogel | Chemically cross-linking | Non-toxic, optimum potential as a new biomaterial for cardiac tissue engineering applications. | 2020 | [114] |
| Alginate scaffolds functionalized with magnetite nanoparticles | Freeze-dry technique | Magnetic alginate scaffolds exposed to an alternating magnetic field create stimulating microenvironments for functional tissue engineering | 2021 | [115] |
| Cardiac ECM-chitosan-gelatin composite | Freezing and lyophilization | High pore size, biodegradable and biocompatible, high cell survival and proliferation. | 2019 | [116] |
| Pathway | Effectors | Functions | References |
|---|---|---|---|
| Hippo | YAP/TAZ | Drives myofibroblast activation and promotes collagen deposition | [154] |
| Rho-A | MRTF-A | Interacts with SRF to regulate the transcription of genes involved in ECM production | [155] |
| Wnt | β-Catenin | Translocates into the nucleus to initiate the transcription of cardiac-related genes | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhejailan, R.S.; Garoffolo, G.; Raveendran, V.V.; Pesce, M. Cells and Materials for Cardiac Repair and Regeneration. J. Clin. Med. 2023, 12, 3398. https://doi.org/10.3390/jcm12103398
Alhejailan RS, Garoffolo G, Raveendran VV, Pesce M. Cells and Materials for Cardiac Repair and Regeneration. Journal of Clinical Medicine. 2023; 12(10):3398. https://doi.org/10.3390/jcm12103398
Chicago/Turabian StyleAlhejailan, Reem Saud, Gloria Garoffolo, Vineesh Vimala Raveendran, and Maurizio Pesce. 2023. "Cells and Materials for Cardiac Repair and Regeneration" Journal of Clinical Medicine 12, no. 10: 3398. https://doi.org/10.3390/jcm12103398
APA StyleAlhejailan, R. S., Garoffolo, G., Raveendran, V. V., & Pesce, M. (2023). Cells and Materials for Cardiac Repair and Regeneration. Journal of Clinical Medicine, 12(10), 3398. https://doi.org/10.3390/jcm12103398







