Simultaneous Comparison of Subxiphoid and Intercostal Wound Pain in the Same Patients Following Thoracoscopic Surgery
Abstract
1. Introduction
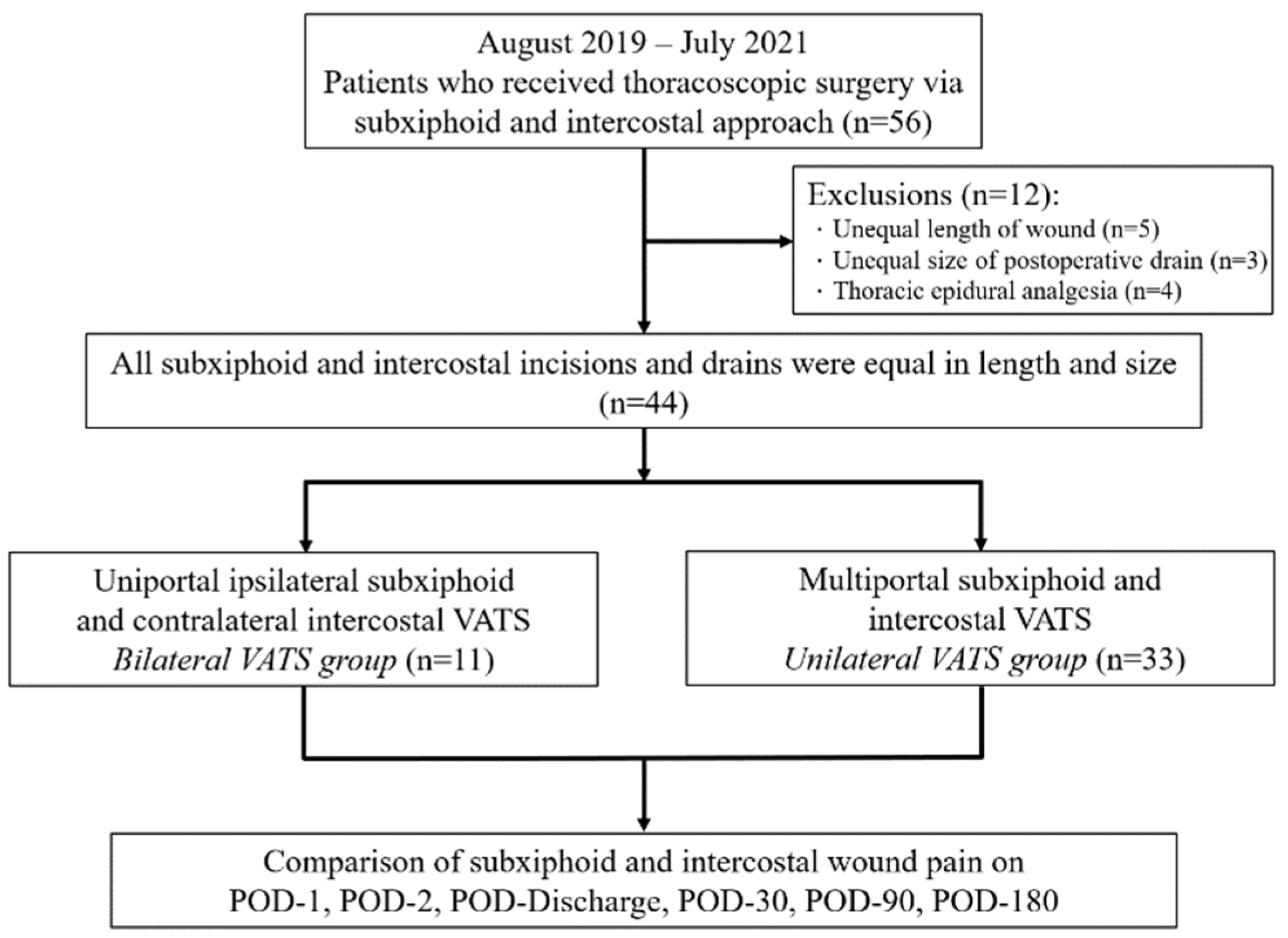
2. Methods
2.1. Study Design
2.2. Operative Technique
2.2.1. Simultaneous Bilateral Uniportal VATS (11 Patients)
2.2.2. Unilateral Multiportal VATS (33 Patients)
2.3. Postoperative Management
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, T.D.; Black, D.; Bannon, P.G.; McCaughan, B.C. Systematic Review and Meta-Analysis of Randomized and Nonrandomized Trials on Safety and Efficacy of Video-Assisted Thoracic Surgery Lobectomy for Early-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2009, 27, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Altorki, N.K.; Sheng, S.; Lee, P.C.; Harpole, D.H.; Onaitis, M.W.; Stiles, B.M.; Port, J.L.; D’Amico, T.A. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J. Thorac. Cardiovasc. Surg. 2010, 139, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Rocco, G. One-port (uniportal) video-assisted thoracic surgical resections—A clear advance. J. Thorac. Cardiovasc. Surg. 2012, 144, S27–S31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirai, K.; Takeuchi, S.; Usuda, J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: A retrospective comparative study of perioperative clinical outcomes. Eur. J. Cardio-Thoracic Surg. 2016, 49 (Suppl. 1), i37–i41. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R.; Shao, F. Comparison of Postoperative Pain and Recovery between Single-Port and Two-Port Thoracoscopic Lobectomy for Lung Cancer. Thorac. Cardiovasc. Surg. 2019, 67, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D.; Nosotti, M.; Bonitta, G.; Mazzucco, A.; Righi, I.; Mendogni, P.; Rosso, L.; Palleschi, A.; Rocco, G.; Crisci, R.; et al. Uniportal and three-portal video-assisted thoracic surgery lobectomy: Analysis of the Italian video-assisted thoracic surgery group database. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Mendogni, P.; Mazzucco, A.; Palleschi, A.; Rosso, L.; Righi, I.; Carrinola, R.; Damarco, F.; Privitera, E.; Fumagalli, J.; Bonitta, G.; et al. Uniportal and three-portal video-assisted thoracic surgery pulmonary lobectomy for early-stage lung cancer (UNIT trial): Study protocol of a single-center randomized trial. Trials 2021, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Bayman, O.E.; Parekh, K.; Keech, J.; Selte, A.; Brennan, T. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017, 126, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Sugimura, H.; Tochii, D.; Kihara, M.; Hattori, Y. Single-Port Thymectomy Through an Infrasternal Approach. Ann. Thorac. Surg. 2012, 93, 334–336. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, B.-Y.; Shih, C.-S.; Liu, Y.-H. Subxiphoid single-incision thoracoscopic left upper lobectomy. J. Thorac. Cardiovasc. Surg. 2014, 148, 3250–3251. [Google Scholar] [CrossRef]
- Wang, B.Y.; Chang, Y.C.; Chang, Y.C.; Wang, K.-M.; Lin, C.-H.; Lin, S.-H.; Lin, W.-C. Thoracoscopic surgery via a single-incision subxiphoid approach is associated with less postoperative pain than single-incision transthoracic or three-incision transthoracic approaches for spontaneous pneumothorax. J. Thorac. Dis. 2016, 8 (Suppl. 3), S272–S278. [Google Scholar]
- Suda, T.; Ishizawa, H.; Nagano, H.; Negi, T.; Kawai, H.; Tochii, D.; Tochii, S.; Hoshikawa, Y. Early outcomes in 147 consecutive cases of subxiphoid single-port thymectomy and evaluation of learning curves. Eur. J. Cardio-Thoracic Surg. 2020, 58 (Suppl. 1), i44–i49. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Zhang, C.; Zhao, D.; Lu, Y.; Wang, Z. The Feasibility and Advantages of Subxiphoid Uniportal Video-Assisted Thoracoscopic Surgery in Pulmonary Lobectomy. World J. Surg. 2019, 43, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Pfeuty, K.; Lenot, B. Multiportal subxiphoid thoracoscopic major pulmonary resections. J. Thorac. Dis. 2019, 11, 2778–2787. [Google Scholar] [CrossRef]
- Cai, H.; Xie, N.; Al Sawalhi, S.; Jiang, L.; Zhu, Y.; Jiang, G.; Zhao, D. Subxiphoid versus intercostal uniportal video-assisted thoracoscopic surgery for bilateral lung resections: A single-institution experience. Eur. J. Cardio-Thoracic Surg. 2020, 57, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.B. Subxiphoid uniportal lobectomy. Eur. J. Cardio-Thoracic Surg. 2016, 50, 1067. [Google Scholar] [CrossRef]
- Chen, J.; Volpi, S.; Ali, J.M.; Aresu, G.; Wu, L.; Chen, Z.; Wang, J.; Chen, B.; Yang, C.; Soultanis, K.M.; et al. Comparison of post-operative pain and quality of life between uniportal subxiphoid and in-tercostal video-assisted thoracoscopic lobectomy. J. Thorac. Dis. 2020, 12, 3582–3590. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sakai, T.; Tsuchiya, T.; Yamasaki, N.; Tagawa, T.; Mine, M.; Shibata, Y.; Nagayasu, T. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur. J. Cardio-Thoracic Surg. 2011, 39, 1033–1039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyazaki, T.; Sakai, T.; Yamasaki, N.; Tsuchiya, T.; Matsumoto, K.; Tagawa, T.; Hatachi, G.; Tomoshige, K.; Mine, M.; Nagayasu, T. Chest tube insertion is one important factor leading to intercostal nerve impairment in thoracic surgery. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.F.; Ravenscroft, A.; Beggs, D.; Duffy, J.P. A questionnaire study investigating the prevalence of the neuropathic compo-nent of chronic pain after thoracic surgery. Eur. J. Cardio-Thoracic Surg. 2006, 29, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.F.; Latter, J.A.; Mahajan, R.; Beggs, F.D.; Duffy, J.P. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur. J. Cardio-Thoracic Surg. 2006, 29, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Steegers, M.A.; Snik, D.M.; Verhagen, A.F.; van der Drift, M.A.; Wilder-Smith, O.H.G. Wilder-Smith. Only half of the chronic pain after thoracic surgery shows a neuropathic com-ponent. J. Pain 2008, 9, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Chang, P.-C.; Chang, S.-J.; Chiang, H.-H.; Li, H.-P.; Chou, S.-H. Simultaneous bilateral thoracoscopic blebs excision reduces contralateral recurrence in patients undergoing operation for ipsilateral primary spontaneous pneumothorax. J. Thorac. Cardiovasc. Surg. 2020, 159, 1120–1127.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, H.W.; Lee, J.Y.; Chiang, H.H.; Li, H.P.; Chang, P.C.; Chou, S.H. Is a Chest Tube Necessary after Video-Assisted Thoracoscopic Mediastinal Tumor Resec-tion? Thorac. Cardiovasc. Surg. 2021, 69, 181–188. [Google Scholar] [PubMed]
- Yoon, S.; Hong, W.-P.; Joo, H.; Jang, D.; Park, S.; Lee, H.-J. Adjuvant chemotherapy as a risk factor for chronic postoperative pain after video-assisted thoracoscopic surgery: A 10-year single-centre retrospective study. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 276–283. [Google Scholar] [CrossRef] [PubMed]






| Case No. | Age | Sex | Procedure Type (Sub vs. ICS) | Port | Drain Size (Sub vs. ICS) (Fr) | Incision Size (Sub vs. ICS) (cm) | Operative Time (Sub vs. ICS) (min) | Blood Loss (Sub vs. ICS) (ml) | Pathology (Sub vs. ICS) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | Wedge resection (RUL vs. LLL) | 1-port | 16/16 | 3.0/3.0 | 50/40 | 5/5 | PLC |
| 2 | 23 | M | Wedge resection (RUL vs. LUL) | 1-port | 24/24 | 2.5/2.5 | 90/70 | 20/10 | Bullae |
| 3 | 56 | M | Wedge resection (RML vs. LLL) | 1-port | 16/16 | 3.0/3.0 | 50/50 | 5/5 | Metastatic RCC |
| 4 | 55 | M | Wedge resection (LLL) vs. lobectomy (RUL) | 1-port | 24/24 | 4.0/4.0 | 70/150 | 20/80 | PLC/Subpleural LN |
| 5 | 18 | M | Wedge resection (RUL vs. LUL) | 1-port | 24/24 | 2.5/2.5 | 40/40 | 20/20 | Bullae |
| 6 | 25 | M | Wedge resection (RUL vs. LUL) | 1-port | 12/12 | 2.5/2.5 | 40/30 | 5/5 | Bullae |
| 7 | 67 | M | Wedge resection (LLL) vs. lobectomy (RUL) | 1-port | 14/14 | 4.0/4.0 | 50/150 | 5/15 | PLC/AIS |
| 8 | 28 | M | Wedge resection (RUL vs. LUL) | 1-port | 12/12 | 2.5/2.5 | 50/50 | 5/5 | Bullae |
| 9 | 54 | F | Wedge resection (RLL vs. LLL) | 1-port | 14/14 | 3.0/3.0 | 60/90 | 5/5 | Sarcoidosis |
| 10 | 57 | F | Wedge resection (RLL vs. LLL) | 1-port | 12/12 | 3.0/3.0 | 70/70 | 5/5 | Tuberculosis |
| 11 | 18 | M | Wedge resection (RUL vs. LUL) | 1-port | 12/12 | 2.0/2.0 | 70/60 | 10/15 | Bullae |
| Case No. | Age | Sex | Procedure Type | Port | Drain Size (Sub vs. ICS) (Fr) | Incision Size (Sub vs. ICS vs. 3rd Port) (cm) | Operative Time (min) | Blood Loss (mL) | Pathology |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | Mediastinal tumor resection | 2-port | No drain | 3.0/3.0 | 90 | 20 | Thymic hyperplasia |
| 2 | 76 | F | Mediastinal tumor resection | 2-port | No drain | 3.0/3.0 | 60 | 5 | Thymoma |
| 3 | 46 | F | Wedge resection (RML) | 2-port | No drain | 2.5/2.5 | 60 | 5 | Metastasizing leiomyoma |
| 4 | 48 | F | Wedge resection (RUL, RLL) | 2-port | 15/15 | 3.0/3.0 | 100 | 20 | Tuberculosis |
| 5 | 71 | F | RLL lobectomy | 3-port | 14 (*) | 4.0/4.0/1.0 | 150 | 15 | Primary lung cancer |
| 6 | 60 | F | RUL lobectomy | 3-port | 20 (*) | 3.0/3.0/1.0 | 170 | 30 | Primary lung cancer |
| 7 | 58 | M | Wedge resection (LUL) | 3-port | 24 (*) | 3.0/3.0/1.0 | 120 | 10 | Metastasis of nasopharyngeal cancer |
| 8 | 57 | M | Pericardial window | 3-port | 12 (*) | 2.5/2.5/0.5 | 90 | 5 | Metastasis of primary lung cancer |
| 9 | 57 | M | RUL lobectomy | 3-port | 28 (*) | 5.0/5.0/1.0 | 210 | 200 | Primary lung cancer |
| 10 | 56 | M | Pericardial window | 3-port | 12 (*) | 2.5/2.5/0.5 | 40 | 5 | Metastasis of primary lung cancer |
| 11 | 64 | M | RLL lobectomy | 3-port | 24 (*) | 4.0/4.0/1.0 | 180 | 60 | Primary lung cancer |
| 12 | 65 | F | Wedge resection (RUL, RLL) | 3-port | 12 (*) | 2.5/2.5/0.5 | 60 | 5 | Metastasis of thymic carcinoma |
| 13 | 52 | M | Wedge resection (RLL) | 2-port | No drain | 2.5/2.5 | 60 | 5 | Metastasis of renal cell carcinoma |
| 14 | 61 | F | Wedge resection (RLL) | 3-port | 15 (*) | 2.5/2.5/0.5 | 60 | 5 | Organizing pneumonia |
| 15 | 54 | M | RUL lobectomy | 2-port | 14/14 | 3.0/3.0 | 170 | 50 | Primary lung cancer |
| 16 | 63 | F | Mediastinal tumor resection | 3-port | 16 (*) | 2.5/2.5/0.5 | 130 | 10 | Thymoma |
| 17 | 56 | F | Mediastinal tumor resection | 3-port | No drain | 2.5/2.5/1.0 | 110 | 5 | Thymic cyst |
| 18 | 63 | M | Mediastinal tumor resection | 3-port | No drain | 3.0/3.0/1.0 | 120 | 20 | Thymolipoma |
| 19 | 58 | F | Mediastinal tumor resection | 3-port | 16 (*) | 2.5/2.5/0.5 | 130 | 10 | Thymoma |
| 20 | 18 | M | Mediastinal tumor resection | 3-port | No drain | 2.5/2.5/1.0 | 100 | 10 | Thymic hyperplasia |
| 21 | 45 | F | Mediastinal tumor resection | 3-port | No drain | 3.0/3.0/1.0 | 120 | 5 | Thymoma |
| 22 | 55 | F | Mediastinal tumor resection | 3-port | No drain | 3.0/3.0/1.0 | 130 | 5 | Thymoma |
| 23 | 44 | M | Mediastinal tumor resection | 3-port | No drain | 3.0/3.0/1.0 | 120 | 10 | Thymoma |
| 24 | 54 | F | Mediastinal tumor resection | 3-port | No drain | 3.0/3.0/1.0 | 100 | 5 | Thymic cyst |
| 25 | 48 | M | Mediastinal tumor resection | 3-port | 16 (*) | 3.0/3.0/1.0 | 150 | 30 | Atypical carcinoid |
| 26 | 52 | M | Mediastinal tumor resection | 3-port | No drain | 2.5/2.5/0.5 | 110 | 10 | Thymoma |
| 27 | 51 | F | Mediastinal tumor resection | 3-port | No drain | 2.5/2.5/0.5 | 90 | 5 | Thymoma |
| 28 | 43 | M | Mediastinal tumor resection | 2-port | No drain | 3.0/3.0 | 110 | 5 | Thymoma |
| 29 | 73 | M | Mediastinal tumor resection | 3-port | 16 (*) | 3.0/3.0/0.5 | 100 | 10 | Angiolipoma |
| 30 | 47 | F | Mediastinal tumor resection | 3-port | 16 (*) | 2.5/2.5/0.5 | 180 | 30 | Thymic carcinoma |
| 31 | 62 | M | Mediastinal tumor resection | 2-port | No drain | 3.0/3.0 | 100 | 5 | Thymic hyperplasia |
| 32 | 39 | M | Mediastinal tumor resection | 3-port | 16 (*) | 2.5/2.5/0.5 | 150 | 10 | Thymoma |
| 33 | 54 | M | Mediastinal tumor resection | 2-port | 14/14 | 3.0/3.0 | 170 | 10 | Thymoma |
| Characteristic | Value |
|---|---|
| Mean age (range), y | 52 (18–76) |
| Gender, % (n) | |
| Male | 59 (26) |
| Female | 41 (18) |
| Smoking (yes), % (n) | 34 (15) |
| Mean BMI (range), kg/m2 | 23 (16.5–30) |
| Pulmonary function test | |
| Mean FEV1 (range), L | 2.5 (1.6–3.7) |
| Mean FEV1 (range), Predicted % | 86 (64–117) |
| Grade I-II complication, % (n) | 11.3 (5) |
| Prolonged air leak (>5 days) | 4.5 (2) |
| Atrial fibrillation | 2.3 (1) |
| Wound allergy | 2.3 (1) |
| Wound poor healing | 2.3 (1) |
| Mean postoperative stay (range), day | 4 (2–9) |
| Median wound length (range), cm | 3.0 (2.0–5.0) |
| Median drain size (range), Fr | 14 (12–28) |
| Median operation time (range), min | 90 (40–240) |
| Median blood loss (range), ml | 10 (5–200) |
| Pain Score | Bilateral VATS (n = 11) | Unilateral VATS (n = 33) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subxiphoid Wound | 95% CI | Intercostal Wound | 95% CI | p-Value | Subxiphoid Wound | 95% CI | Intercostal Wound | 95% CI | p-Value | |
| POD-1 | 5.1 ± 1.4 | (4.1–6.0) | 2.5 ± 1.4 | (1.5–3.4) | 0.0003 | 1.8 ± 1.5 | (1.3–2.4) | 2.0 ± 1.6 | (1.4–2.5) | 0.52 |
| POD-2 | 3.6 ± 1.7 | (2.5–4.8) | 1.6 ± 0.8 | (1.1–2.2) | 0.001 | 1.2 ± 1.1 | (0.8–1.6) | 1.2 ± 1.4 | (0.7–1.7) | 0.72 |
| POD-Discharge | 1.9 ± 1.4 | (1.0–2.8) | 1.1 ± 0.5 | (0.7–1.5) | 0.03 | 0.6 ± 0.7 | (0.4–0.9) | 0.8 ± 1.0 | (0.5–1.2) | 0.16 |
| POD-30 | 0.4 ± 0.9 | (0.0–1.0) | 0.6 ± 0.9 | (0.0–1.3) | 0.49 | 0.3 ± 0.8 | (0.0–0.6) | 0.5 ± 1.1 | (0.0–0.9) | 0.32 |
| POD-90 | 0 ± 0 | (0.0–0.0) | 0.4 ± 0.5 | (0.0–0.7) | 0.03 | 0.1 ± 0.3 | (0.0–0.2) | 0.5 ± 1.1 | (0.1–0.9) | 0.03 |
| POD-180 | 0 ± 0 | (0.0–0.0) | 0.2 ± 0.4 | (0.0–0.5) | 0.16 | 0 ± 0 | (0.0–0.0) | 0.1 ± 0.3 | (0.0–0.2) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-W.; Chou, S.-H.; Chou, A.; Kao, C.-N. Simultaneous Comparison of Subxiphoid and Intercostal Wound Pain in the Same Patients Following Thoracoscopic Surgery. J. Clin. Med. 2022, 11, 2254. https://doi.org/10.3390/jcm11082254
Liu Y-W, Chou S-H, Chou A, Kao C-N. Simultaneous Comparison of Subxiphoid and Intercostal Wound Pain in the Same Patients Following Thoracoscopic Surgery. Journal of Clinical Medicine. 2022; 11(8):2254. https://doi.org/10.3390/jcm11082254
Chicago/Turabian StyleLiu, Yu-Wei, Shah-Hwa Chou, Andre Chou, and Chieh-Ni Kao. 2022. "Simultaneous Comparison of Subxiphoid and Intercostal Wound Pain in the Same Patients Following Thoracoscopic Surgery" Journal of Clinical Medicine 11, no. 8: 2254. https://doi.org/10.3390/jcm11082254
APA StyleLiu, Y.-W., Chou, S.-H., Chou, A., & Kao, C.-N. (2022). Simultaneous Comparison of Subxiphoid and Intercostal Wound Pain in the Same Patients Following Thoracoscopic Surgery. Journal of Clinical Medicine, 11(8), 2254. https://doi.org/10.3390/jcm11082254






