Biventricular Myocardial Strain Analysis in Patients with Pulmonary Arterial Hypertension Using Cardiac Magnetic Resonance Tissue-Tracking Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
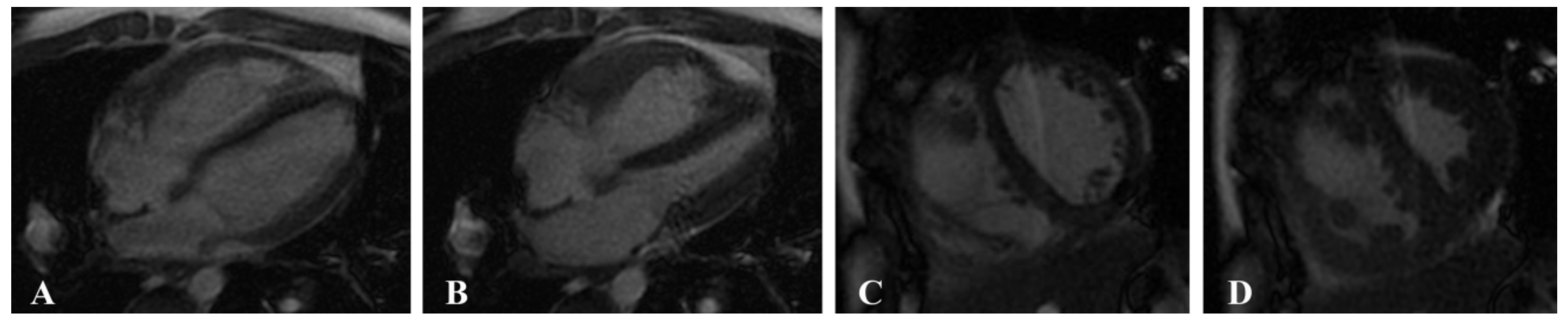
2.3. Image Postprocessing
2.3.1. Cardiac Function Parameters
2.3.2. Myocardial Strain Parameters
2.4. Statistical Analysis
2.5. Repeatability Test
3. Results
3.1. General Information
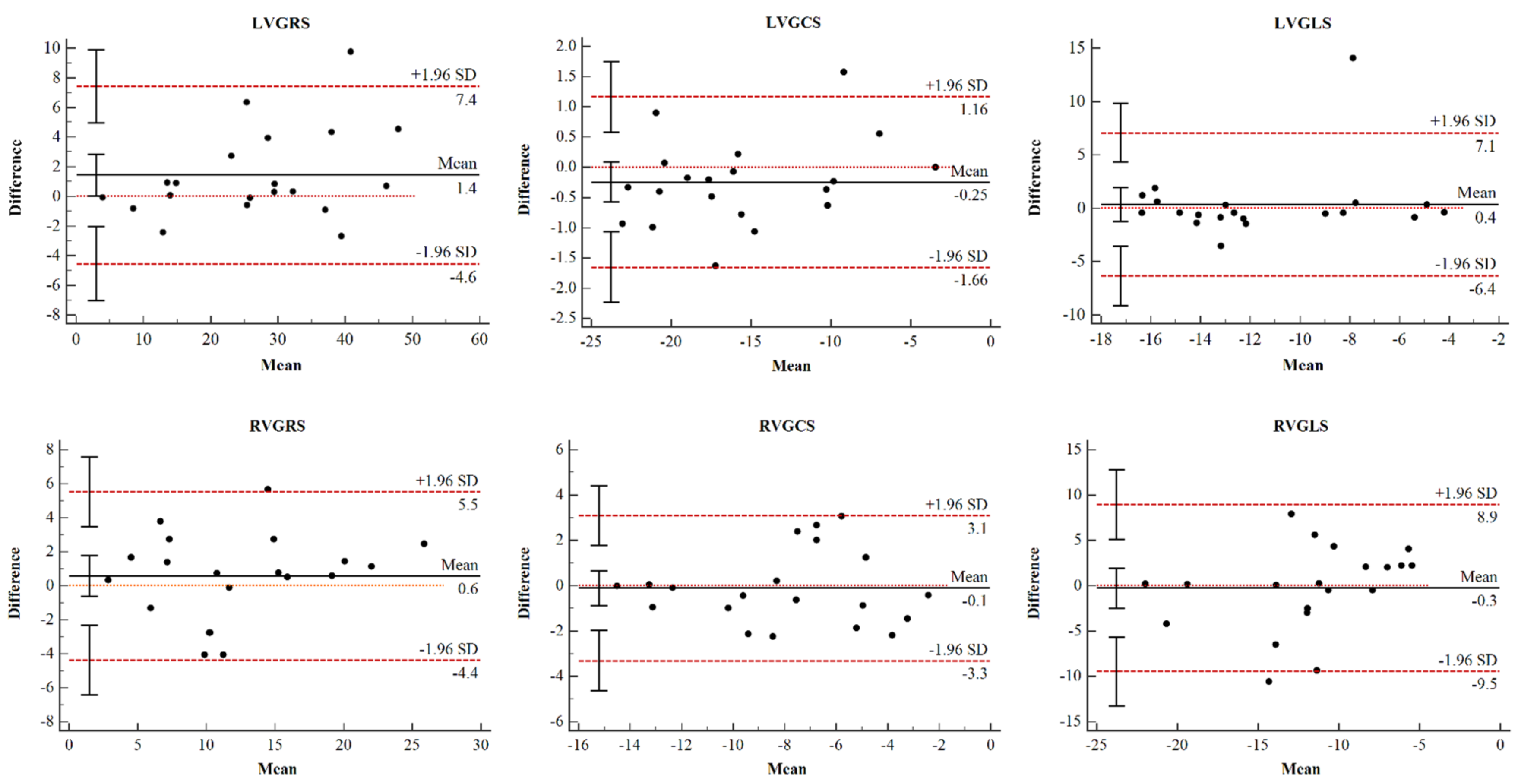
3.2. Reproducibility Analysis
3.3. Strain Analysis in the PAH and Control Groups
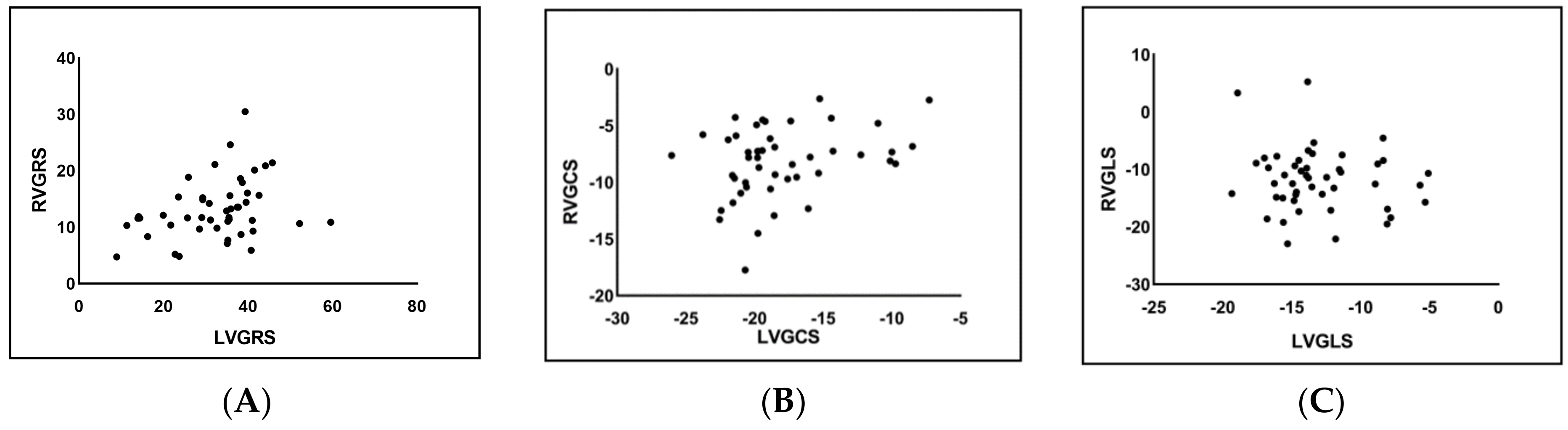
3.4. Correlation Analysis of LV and RV Strain in the PAH Group
3.5. RV Myocardial Strain Analysis in PAH Patients with Preserved and Reduced RV Function
3.6. LV Myocardial Strain Analysis in PAH Patients with Preserved and Reduced LV Function
4. Discussion
4.1. Feasibility and Repeatability of CMR-TT
4.2. Biventricular Myocardial Strain Analysis in PAH Patients
4.3. Strain Analysis of PAH Patients with Preserved RV Function
4.4. Strain Analysis of PAH Patients with Preserved LV Function
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D42–D50. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, M.C.; Kind, T.; Marcus, J.T.; Mauritz, G.J.; Heymans, M.W.; Bogaard, H.J.; Boonstra, A.; Marques, K.M.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J. Am. Coll. Cardiol. 2011, 58, 2511–2519. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef]
- Junqueira, F.P.; Sasdeli Neto, R. Cardiac magnetic resonance imaging and clinical prognosis in pulmonary arterial hypertension. Radiol. Bras. 2019, 52, 5–6. [Google Scholar] [CrossRef]
- Goten, C.; Usui, S.; Takashima, S.I.; Inoue, O.; Okada, H.; Shimojima, M.; Sakata, K.; Kawashiri, M.; Kaneko, S.; Takamura, M. Circulating nerve growth factor receptor positive cells are associated with severity and prognosis of pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 2045894021990525. [Google Scholar] [CrossRef]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D34–D41. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev. Esp. Cardiol. 2016, 69, 177. [Google Scholar] [CrossRef]
- Melillo, C.A.; Lane, J.E.; Aulak, K.S.; Almoushref, A.; Dweik, R.A.; Tonelli, A.R. Repeatability of Pulmonary Pressure Measurements in Patients with Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2020, 17, 1028–1030. [Google Scholar] [CrossRef]
- Saito, T.; Kasai, H.; Sugiura, T.; Takahashi, Y.; Tajima, H.; Shigeta, A.; Sakao, S.; Tanabe, N.; Tatsumi, K. Effects of pulmonary endarterectomy on pulmonary hemodynamics in chronic thromboembolic pulmonary hypertension, evaluated by interventricular septum curvature. Pulm. Circ. 2020, 10, 204589401989750. [Google Scholar] [CrossRef]
- Dennis, M.; Ugander, M.; Kozor, R.; Puranik, R. Cardiovascular Magnetic Resonance Imaging of Inherited Heart Conditions. Heart Lung Circ. 2020, 29, 584–593. [Google Scholar] [CrossRef]
- Salerno, M.; Sharif, B.; Arheden, H.; Kumar, A.; Axel, L.; Li, D.; Neubauer, S. Recent Advances in Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e003951. [Google Scholar] [CrossRef]
- Swift, A.J.; Capener, D.; Johns, C.; Hamilton, N.; Rothman, A.; Elliot, C.; Condliffe, R.; Charalampopoulos, A.; Rajaram, S.; Lawrie, A.; et al. Magnetic Resonance Imaging in the Prognostic Evaluation of Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2017, 196, 228–239. [Google Scholar] [CrossRef]
- De Siqueira, M.E.; Pozo, E.; Fernandes, V.R.; Sengupta, P.P.; Modesto, K.; Gupta, S.S.; Barbeito-Caamano, C.; Narula, J.; Fuster, V.; Caixeta, A.; et al. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2016, 18, 39. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Kim, S.M.; Park, S.-J.; Cho, E.J.; Kim, E.K.; Chang, S.-A.; Lee, S.-C.; Choe, Y.H.; Park, S.W. Assessment of reverse remodeling predicted by myocardial deformation on tissue tracking in patients with severe aortic stenosis: A cardiovascular magnetic resonance imaging study. J. Cardiovasc. Magn. Reson. 2017, 19, 80. [Google Scholar] [CrossRef]
- Williams, L.K.; Forero, J.F.; Popovic, Z.B.; Phelan, D.; Delgado, D.; Rakowski, H.; Wintersperger, B.J.; Thavendiranathan, P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J. Cardiovasc. Magn. Reson. 2017, 19, 61. [Google Scholar] [CrossRef]
- Haeck, M.L.A.; Scherptong, R.W.C.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Vliegen, H.W.; Delgado, V. Prognostic Value of Right Ventricular Longitudinal Peak Systolic Strain in Patients with Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2012, 5, 628–636. [Google Scholar] [CrossRef]
- Mirea, O.; Duchenne, J.; Voigt, J.-U. Recent advances in echocardiography: Strain and strain rate imaging. F1000Research 2016, 5, 787. [Google Scholar] [CrossRef][Green Version]
- Truong, V.T.; Safdar, K.S.; Kalra, D.K.; Gao, X.; Ambach, S.; Taylor, M.D.; Moore, R.; Taylor, R.J.; Germann, J.; Toro-Salazar, O.; et al. Cardiac magnetic resonance tissue tracking in right ventricle: Feasibility and normal values. Magn. Reson. Imaging 2017, 38, 189–195. [Google Scholar] [CrossRef]
- Xie, L.-J.; Dong, Z.-H.; Yang, Z.-G.; Deng, M.-Y.; Gao, Y.; Jiang, L.; Hu, B.-Y.; Liu, X.; Ren, Y.; Xia, C.-C.; et al. Assessment of left ventricular deformation in patients with type 2 Diabetes mellitus by cardiac magnetic resonance tissue tracking. Sci. Rep. 2020, 10, 13126. [Google Scholar] [CrossRef]
- D’Andrea, A.; Stanziola, A.; D’Alto, M.; Di Palma, E.; Martino, M.; Scarafile, R.; Molino, A.; Rea, G.; Maglione, M.; Calabro, R.; et al. Right ventricular strain: An independent predictor of survival in idiopathic pulmonary fibrosis. Int. J. Cardiol. 2016, 222, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Mangion, K.; McComb, C.; Auger, D.A.; Epstein, F.H.; Berry, C. Magnetic Resonance Imaging of Myocardial Strain After Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review. Circ. Cardiovasc. Imaging 2017, 10, e006498. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Narula, J. Cardiac strain as a universal biomarker: Interpreting the sounds of uneasy heart muscle cells. JACC Cardiovasc. Imaging 2014, 7, 534–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kallianos, K.; Brooks, G.C.; Mukai, K.; Seguro de Carvalho, F.; Liu, J.; Naeger, D.M.; De Marco, T.; Ordovas, K.G. Cardiac Magnetic Resonance Evaluation of Left Ventricular Myocardial Strain in Pulmonary Hypertension. Acad. Radiol. 2018, 25, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Padervinskiene, L.; Krivickiene, A.; Hoppenot, D.; Miliauskas, S.; Basevicius, A.; Nedzelskiene, I.; Jankauskas, A.; Simkus, P.; Ereminiene, E. Prognostic Value of Left Ventricular Function and Mechanics in Pulmonary Hypertension: A Pilot Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina 2019, 55, 73. [Google Scholar] [CrossRef]
- Lindholm, A.; Hesselstrand, R.; Rådegran, G.; Arheden, H.; Ostenfeld, E. Decreased biventricular longitudinal strain in patients with systemic sclerosis is mainly caused by pulmonary hypertension and not by systemic sclerosis per se. Clin. Physiol. Funct. Imaging 2019, 39, 215–225. [Google Scholar] [CrossRef]
- Lin, A.C.W.; Seale, H.; Hamilton-Craig, C.; Morris, N.R.; Strugnell, W. Quantification of biventricular strain and assessment of ventriculo-ventricular interaction in pulmonary arterial hypertension using exercise cardiac magnetic resonance imaging and myocardial feature tracking. J. Magn. Reson. Imaging JMRI 2019, 49, 1427–1436. [Google Scholar] [CrossRef]
- Puwanant, S.; Park, M.; Popović, Z.B.; Tang, W.H.; Farha, S.; George, D.; Sharp, J.; Puntawangkoon, J.; Loyd, J.E.; Erzurum, S.C.; et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010, 121, 259–266. [Google Scholar] [CrossRef]
- Padervinskiene, L.; Hoppenot, D.; Krivickiene, A.; Gumauskiene, B.; Nedzelskiene, I.; Simkus, P.; Miliauskas, S.; Jankauskas, A.; Basevicius, A.; Ereminiene, E. Identification of Cardiac MRI and Bio-Marker Thresholds for One-Year Survival in Pre-Capillary Pulmonary Hypertension: Prospective Study. Medicina 2020, 56, 167. [Google Scholar] [CrossRef]
- Berry, C.; Mangion, K.; Pathan, F. Spotlight on Strain Following Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1445–1447. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Li, S.; Zhao, Y.; Li, P. Age- and gender-related normal references of right ventricular strain values by tissue tracking cardiac magnetic resonance: Results from a Chinese population. Quant. Imaging Med. Surg. 2019, 9, 1441–1450. [Google Scholar] [CrossRef]

| PAH Group (n = 47) | Healthy Control Group (n = 32) | p | |
|---|---|---|---|
| Age (years) | 49.32 ± 14.23 | 53.13 ± 15.06 | 0.258 |
| Gender (male/female) | 9/38 | 17/15 | <0.001 |
| Height (cm) | 164.00 ± 6.06 | 167.19 ± 7.28 | 0.059 |
| Weight (kg) | 60.26 ± 8.64 | 61.78 ± 10.27 | 0.167 |
| BMI (kg/m2) | 22.28 ± 1.69 | 21.94 ± 2.13 | 0.115 |
| Heart rate (bpm) | 69.68 ± 5.28 | 71.12 ± 5.92 | 0.561 |
| LVEF % | 62.29 ± 10.97 | 67.84 ± 6.90 | 0.013 |
| LVEDV (mL) | 85.60 ± 32.88 | 116.61 ± 24.70 | <0.001 |
| LVESV (mL) | 34.31 ± 26.20 | 37.74 ± 12.04 | 0.492 |
| RVEF % | 29.80 ± 8.30 | 47.92 ± 7.04 | <0.001 |
| RVEDV (mL) | 132.72 ± 43.41 | 106.16 ± 27.13 | 0.003 |
| RVESV (mL) | 94.74 ± 36.93 | 54.70 ± 16.53 | <0.001 |
| ICC | 95% CI | p | |
|---|---|---|---|
| LVGRS % | 0.976 | 0.932–0.991 | <0.01 |
| LVGCS % | 0.985 | 0.956–0.994 | <0.01 |
| LVGLS % | 0.819 | 0.540–0.929 | <0.01 |
| RVGRS % | 0.957 | 0.894–0.983 | <0.01 |
| RVGCS % | 0.947 | 0.867–0.979 | <0.01 |
| RVGLS % | 0.759 | 0.382–0.905 | <0.01 |
| PAH Group (n = 47) | Healthy Control Group (n = 32) | p | |
|---|---|---|---|
| LVGRS % | 32.37 ± 10.60 | 36.31 ± 7.92 | 0.077 |
| LVGLS % | −13.04 ± 3.49 | −16.79 ± 2.86 | <0.001 |
| LVGCS % | −18.01 ± 4.21 | −19.91 ± 2.90 | 0.027 |
| RVGRS % | 13.18 ± 5.21 | 24.33 ± 7.98 | <0.001 |
| RVGLS % | −11.81 ± 5.46 | −20.08 ± 6.41 | <0.001 |
| RVGCS % | −8.19 ± 3.10 | −13.30 ± 3.61 | <0.001 |
| RVEF < 40% Group (n = 38) | RVEF ≥ 40% Group (n = 9) | Healthy Control Group (n = 32) | p | |
|---|---|---|---|---|
| RVGRS % | 12.31 ± 4.80 b | 16.85 ± 5.57 b | 24.33 ± 7.98 | <0.001 |
| RVGLS % | −11.13 ± 5.62 b | −14.70 ± 3.68 b | −20.08 ± 6.41 | <0.001 |
| RVGCS % | −7.71 ± 2.90 ab | −10.22 ± 3.26 b | −13.30 ± 3.61 | <0.001 |
| RVGCS-b % | −7.38 ± 3.85 | −7.58 ± 6.33 | −7.45 ± 8.52 | 0.996 |
| RVGCS-m % | −8.05 ± 4.40 ab | −11.49 ± 3.04 b | −15.36 ± 3.06 | <0.001 |
| RVGCS-a % | −6.38 ± 2.62 ab | −13.43 ± 4.18 b | −19.08 ± 5.49 | <0.001 |
| RVGRS-b % | 12.37 ± 4.28 b | 14.34 ± 8.22 b | 18.48 ± 7.53 | 0.001 |
| RVGRS-m % | 13.26 ± 6.32 b | 18.34 ± 5.73 b | 27.99 ± 11.90 | <0.001 |
| RVGRS-a % | 14.99 ± 4.94 b | 23.20 ± 8.96 b | 38.07 ± 18.69 | <0.001 |
| LVEF < 60% Group (n = 15) | LVEF ≥ 60% Group (n = 32) | Healthy Control Group (n = 32) | p | |
|---|---|---|---|---|
| LVGRS % | 21.97 ± 7.41 ab | 37.24 ± 8.06 | 36.31 ± 7.92 | <0.001 |
| LVGLS % | −10.51 ± 3.14 ab | −14.23 ± 3.01 b | −16.79 ± 2.86 | <0.001 |
| LVGCS % | −14.01 ± 3.76 ab | −19.89 ± 2.92 | −19.91 ± 2.90 | <0.001 |
| LVGCS-b % | −14.04 ± 3.46 ab | −18.74 ± 2.74 | −18.81 ± 2.75 | <0.001 |
| LVGCS-m % | −13.64 ± 3.63 ab | −19.26 ± 3.53 | −19.80 ± 3.06 | <0.001 |
| LVGCS-a % | −17.33 ± 5.42 ab | −24.82 ± 2.96 | −24.43 ± 4.29 | <0.001 |
| LVGRS-b % | 22.10 ± 7.00 ab | 33.57 ± 7.24 | 33.51 ± 7.31 | <0.001 |
| LVGRS-m % | 20.59 ± 6.85 ab | 34.84 ± 9.66 | 35.34 ± 9.04 | <0.001 |
| LVGRS-a % | 29.77 ± 14.37 ab | 57.17 ± 15.06 | 53.64 ± 18.10 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Li, S.; Cui, L.; Zhu, K.; Huo, H.; Liu, T. Biventricular Myocardial Strain Analysis in Patients with Pulmonary Arterial Hypertension Using Cardiac Magnetic Resonance Tissue-Tracking Technology. J. Clin. Med. 2022, 11, 2230. https://doi.org/10.3390/jcm11082230
Cao J, Li S, Cui L, Zhu K, Huo H, Liu T. Biventricular Myocardial Strain Analysis in Patients with Pulmonary Arterial Hypertension Using Cardiac Magnetic Resonance Tissue-Tracking Technology. Journal of Clinical Medicine. 2022; 11(8):2230. https://doi.org/10.3390/jcm11082230
Chicago/Turabian StyleCao, Jibin, Simiao Li, Lingling Cui, Kexin Zhu, Huaibi Huo, and Ting Liu. 2022. "Biventricular Myocardial Strain Analysis in Patients with Pulmonary Arterial Hypertension Using Cardiac Magnetic Resonance Tissue-Tracking Technology" Journal of Clinical Medicine 11, no. 8: 2230. https://doi.org/10.3390/jcm11082230
APA StyleCao, J., Li, S., Cui, L., Zhu, K., Huo, H., & Liu, T. (2022). Biventricular Myocardial Strain Analysis in Patients with Pulmonary Arterial Hypertension Using Cardiac Magnetic Resonance Tissue-Tracking Technology. Journal of Clinical Medicine, 11(8), 2230. https://doi.org/10.3390/jcm11082230





