Impact of the COVID-19 Pandemic on Maternal Well-Being during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Aims of the Study
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
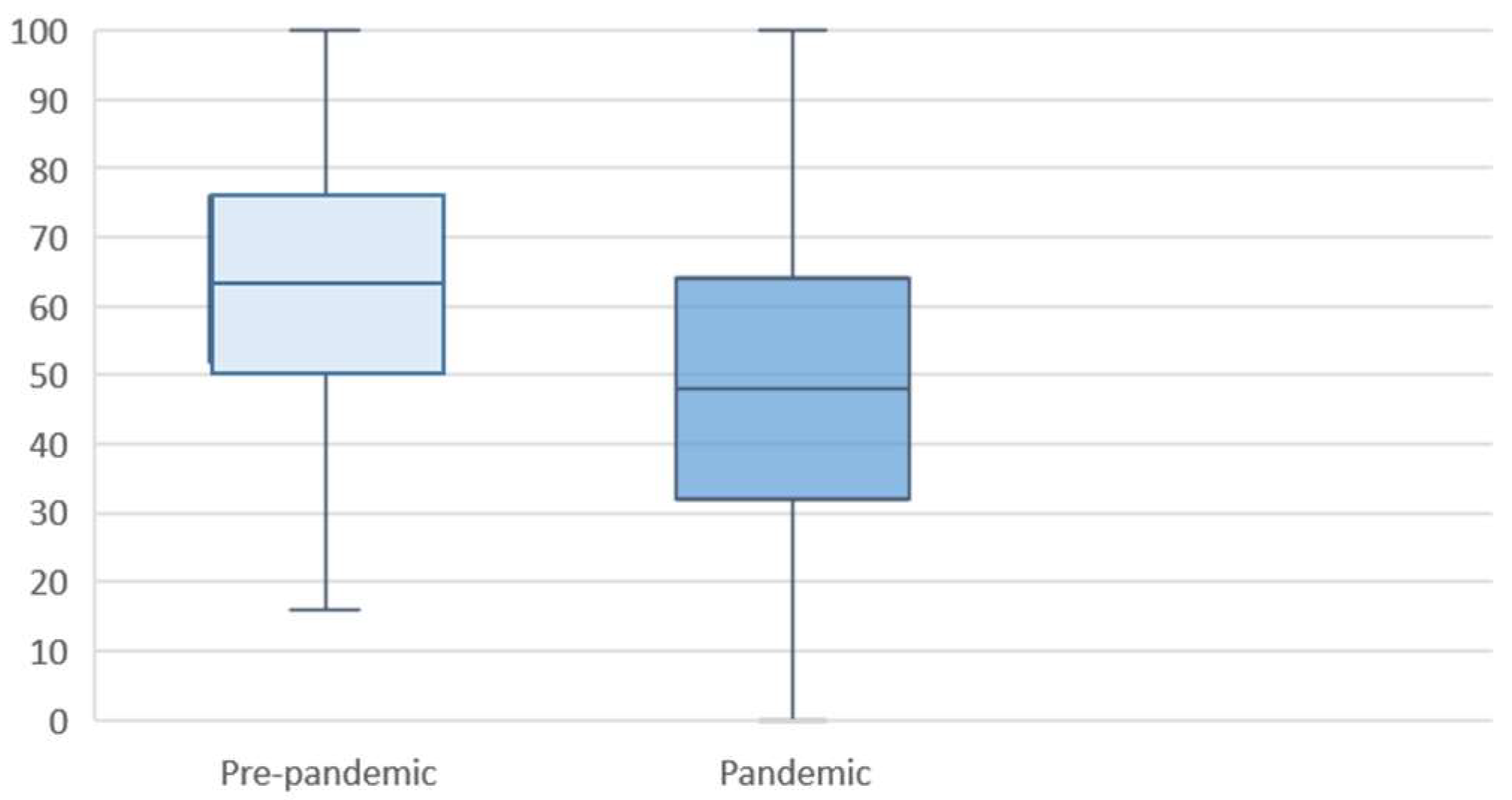
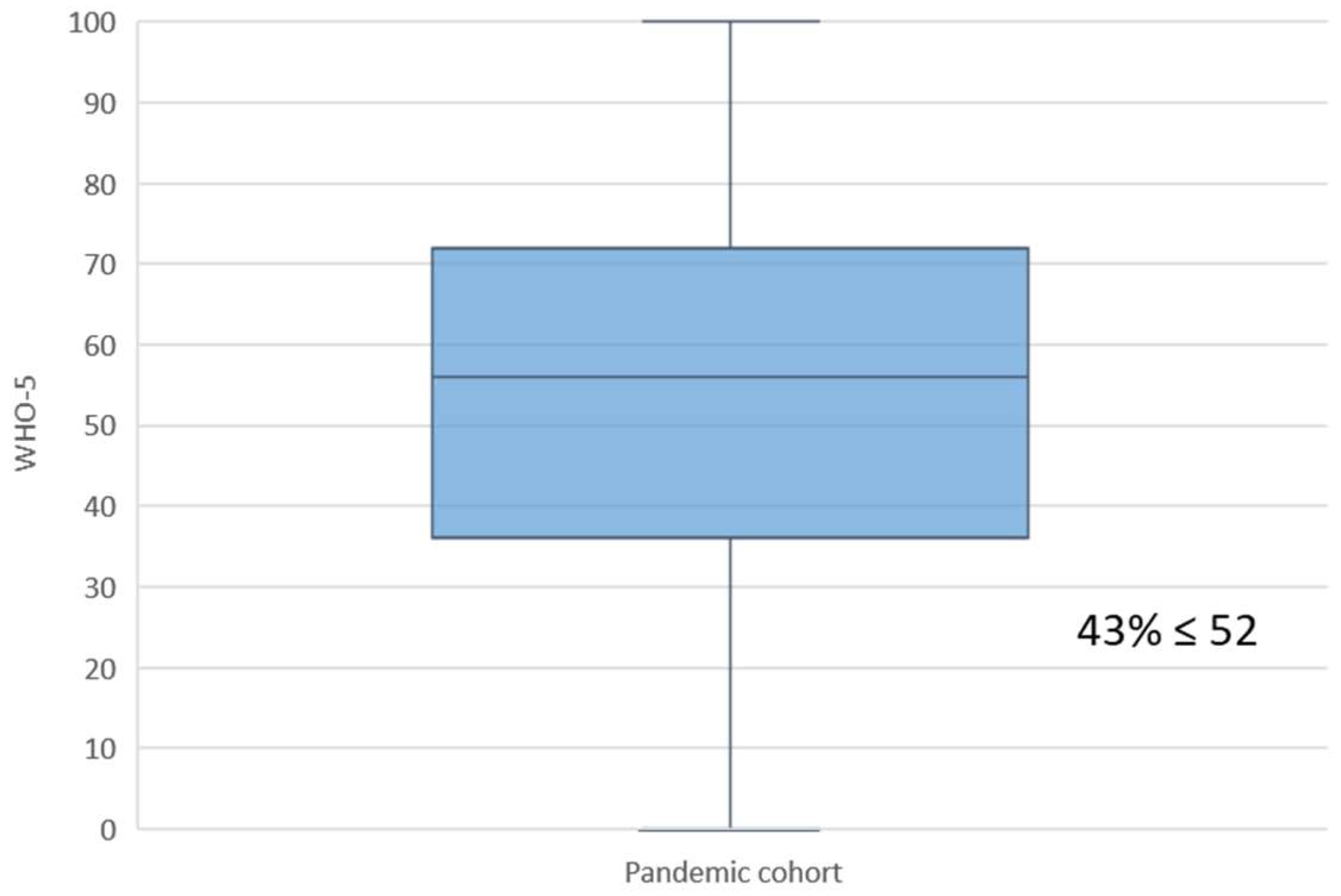
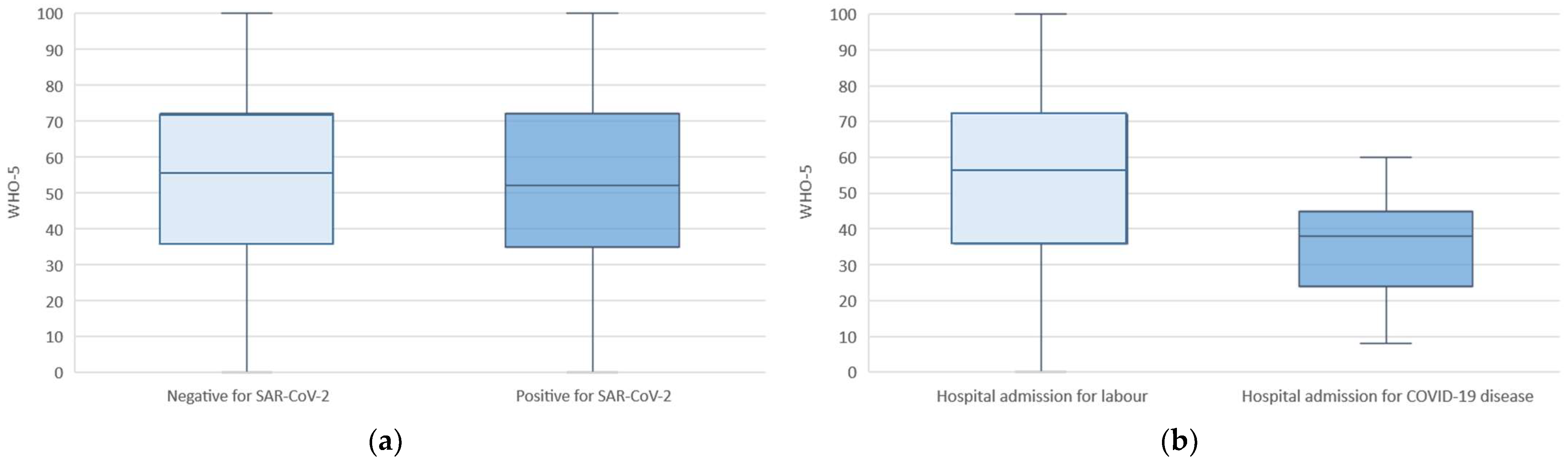
3.2. Maternal Well-Being
3.3. Lockdown Characteristics
4. Discussion
4.1. Main Findings
4.2. Clinical Relevance
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. COVID-19 Evaluation
Appendix A.2. Sample Collection
Appendix B
| Characteristics | Pre-Pandemic (n = 345) | Pandemic (n = 1320) | p-Value |
|---|---|---|---|
| Ethnicity | |||
| White | 279 (80.9%) | 858 (65%) | <0.001 |
| Latin American | 49 (14.2%) | 297 (22.5%) | 0.001 |
| Black | 6 (1.7%) | 23 (1.7%) | 0.997 |
| Asian | 6 (1.7%) | 81 (6.1%) | 0.001 |
| Others | 5 (1.4%) | 61 (4.6%) | 0.007 |
| Tobacco use during pregnancy | 27 (7.8%) | 127 (9.6%) | 0.305 |
| Pre-pregnancy BMI (kg/h2) | 23.8 (4.8) | 24.1 (4.7) | 0.29 |
| Medical history | |||
| Obesity (BMI > 30) | 39 (11.3%) | 157 (11.9%) | 0.762 |
| Psychiatric disorders * | 15 (4.3%) | 28 (2.1%) | 0.020 |
| Thyroid diseases | 31 (9%) | 91 (6.9%) | 0.184 |
| Obstetric history | |||
| Nulliparous | 203 (58.8%) | 725 (54.9%) | 0.192 |
| Characteristics | Total Cohort (n = 480) | WHO-5 ≤ 52 | WHO-5 >52 | p-Value |
|---|---|---|---|---|
| SARS-CoV-2 diagnosis by laboratory test | 0.079 | |||
| Yes | 7 (1.5%) | 10 (3.4%) | 2 (1%) | |
| No | 473 (98.5%) | 287 (96.6%) | 207 (99%) | |
| Contact with a symptomatic SARS-CoV-2 person | 0.098 | |||
| Yes | 42 (8.8%) | 21 (7%) | 24 (11.2%) | |
| No | 438 (91.3%) | 278 (93%) | 190 (88.8%) | |
| Know someone diagnosed by SARS-CoV-2 | 0.247 | |||
| Yes | 129 (26.9%) | 74 (24.4%) | 62 (29%) | |
| No | 351 (73.1%) | 229 (75.6%) | 152 (71%) | |
| Degree of concern about SARS-CoV-2 epidemic | 0.088 | |||
| I’m very worried | 192 (40%) | 112 (37.2%) | 94 (44.1%) | |
| I’m quite worried | 222 (46.3%) | 141 (46.8%) | 97 (45.5%) | |
| I’m a little worried | 59 (12.3%) | 45 (15%) | 18 (8.5%) | |
| Don’t care | 7 (1.5%) | 3 (1%) | 4 (1.9%) | |
| Worry of getting the disease yourself or a family member | 0.537 | |||
| I’m very worried | 279 (58.1%) | 170 (56.1%) | 133 (62.1%) | |
| I’m quite worried | 159 (33.1%) | 107 (35.3%) | 63 (29.4%) | |
| I’m a little worried | 40 (8.3%) | 25 (8.3%) | 17 (7.9%) | |
| Don’t care | 2 (0.4%) | 1 (0.3%) | 1 (0.5%) | |
| Effect on the pregnancy and fetus concerns | 0.220 | |||
| I’m very worried | 332 (69.2%) | 202 (66.9%) | 156 (72.9%) | |
| I’m quite worried | 84 (17.5%) | 58 (19.2%) | 32 (15%) | |
| I’m a little worried | 53 (11%) | 33 (10.9%) | 24 (11.2%) | |
| Don’t care | 11 (2.3%) | 9 (3%) | 2 (0.9%) | |
| Personal economic concern | 0.944 | |||
| I’m very worried | 226 (47.1%) | 146 (48.2%) | 102 (47.7%) | |
| I’m quite worried | 148 (30.8%) | 88 (29%) | 66 (30.8%) | |
| I’m a little worried | 86 (17.9%) | 55 (18.2%) | 38 (17.8%) | |
| Don’t care | 20 (4.2%) | 14 (4.6%) | 8 (3.7%) | |
| Impact on global economy concerns | 0.110 | |||
| I’m very worried | 199 (41.5%) | 124 (40.9%) | 93 (43.5%) | |
| I’m quite worried | 198 (41.3%) | 116 (38.3%) | 94 (43.9%) | |
| I’m a little worried | 72 (15%) | 55 (18.2%) | 24 (11.2%) | |
| Don’t care | 11 (2.3%) | 8 (2.6%) | 3 (1.4%) | |
| Excessive worrying | 0.092 | |||
| Yes | 41 (8.5%) | 36 (11.9%) | 14 (6.5%) | |
| No | 439 (91.5%) | 267 (88.1%) | 200 (93.5%) | |
| Does the pregnant woman have enough information regarding the effects of the virus on pregnancy and the fetus | 0.332 | |||
| Yes | 216 (45%) | 141 (47-2%) | 89 (41.6%) | |
| No | 264 (55%) | 160 (52.8%) | 125 (58.4%) | |
| Isolation in primary residence | 0.515 | |||
| Yes | 448 (93.3%) | 277 (91.4%) | 199 (93%) | |
| No | 32 (6.7%) | 26 (8.6%) | 25 (7%) | |
| People at risk living at home | 0.548 | |||
| Yes | 46 (9.6%) | 27 (8.9%) | 23 (10.8%) | |
| No | 434 (90.4%) | 275 (91%) | 189 (89.2%) | |
| Terrace or garden at home | 0.809 | |||
| Yes | 251 (52.3%) | 158 (53.2%) | 111 (52.1%) | |
| No | 229 (47.7%) | 139 (46.8%) | 102 (47.9%) | |
| Work | 0.748 | |||
| No | 419 (87.3%) | 266 (88.1%) | 184 (86%) | |
| Yes, from home | 51 (10.6%) | 29 (9.6%) | 25 (11.7%) | |
| Yes, at my usual place of work | 10 (2.1%) | 7 (2.3%) | 5 (2.3%) | |
| How many times a week does she go out | 0.352 | |||
| Never | 162 (33.8%) | 97 (32%) | 78 (36.4%) | |
| One or two times a week | 232 (48.3%) | 146 (48.2%) | 106 (49.5%) | |
| Between three and five times a week | 52 (10.8%) | 36 (11.9%) | 19 (8.9%) | |
| Six or more times a week | 34 (7.1%) | 24 (7.9%) | 11 (5.1%) | |
| Coping with isolation | <0.001 | |||
| Very well | 94 (19.6%) | 69 (23.1%) | 28 (13.1%) | |
| Pretty well | 309 (64.4%) | 197 (65.9%) | 136 (63.8%) | |
| Poorly | 68 (14.2%) | 29 (9.7%) | 42 (19.7%) | |
| Very poorly | 9 (1.9%) | 4 (1.3%) | 7 (3.3%) | |
| Mental health before the pandemic | 0.069 | |||
| Excellent | 106 (26.6%) | 75 (29.8%) | 38 (21.5%) | |
| Very good | 180 (45.2%) | 115 (45.6%) | 81 (45.8%) | |
| Good | 97 (24.4%) | 56 (22.2%) | 46 (26%) | |
| Regular | 11 (2.8%) | 4 (1.6%) | 10 (5.6%) | |
| Bad | 4 (1%) | 2 (0.8%) | 2 (1.1%) |
| Characteristics | Total Cohort (n = 1320) |
|---|---|
| SARS-CoV-2 positive (RT-PCR and/or Ab) | 202 (15.3%) |
| First trimester | 82 (40.6%) |
| Third trimester | 120 (59.4%) |
| RT-PCRa positive | 26 (3%) |
| Ab for SARS-CoV-2 infection IgM/A/G | |
| Negative | 1120 (84.8%) |
| Positive | 200 (15.2%) |
References
- BOE.es-BOE-A-2020-5767 Real Decreto 555/2020, de 5 de Junio, por el que se Prorroga el Estado de Alarma Declarado por el Real Decreto 463/2020, de 14 de Marzo, por el que se Declara el Estado de Alarma para la Gestión de la Situación de Crisis Sanitaria Ocasionada por el COVID-19. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2020-5767 (accessed on 31 May 2021).
- Liu, N.; Zhang, F.; Wei, C.; Jia, Y.; Shang, Z.; Sun, L.; Wu, L.; Sun, Z.; Zhou, Y.; Wang, Y.; et al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: Gender differences matter. Psychiatry Res. 2020, 287, 112921. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Crovetto, F.; Crispi, F.; Llurba, E.; Pascal, R.; Larroya, M.; Trilla, C.; Camacho, M.; Medina, C.; Dobaño, C.; Gomez-Roig, M.D.; et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Infection on Pregnancy Outcomes: A Population-based Study. Clin. Infect. Dis. 2021, 73, 1768–1775. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 226, 68–89.e3. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. Obstet. Gynecol. Surv. 2022, 77, 80–82. [Google Scholar] [CrossRef]
- Buekens, P.; Alger, J.; Bréart, G.; Cafferata, M.L.; Harville, E.; Tomasso, G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob. Health 2020, 8, e877–e878. [Google Scholar] [CrossRef]
- BOE.es-BOE-A-2020-3692 Real Decreto 463/2020, de 14 de Marzo, por el que se Declara el Estado de Alarma para la Gestión de la Situación de Crisis Sanitaria Ocasionada por el COVID-19. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2020-3692 (accessed on 28 July 2021).
- Lebel, C.; MacKinnon, A.; Bagshawe, M.; Tomfohr-Madsen, L.; Giesbrecht, G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J. Affect. Disord. 2020, 277, 5–13. [Google Scholar] [CrossRef]
- Khoury, J.E.; Atkinson, L.; Bennett, T.; Jack, S.M.; Gonzalez, A. COVID-19 and mental health during pregnancy: The importance of cognitive appraisal and social support. J. Affect. Disord. 2021, 282, 1161–1169. [Google Scholar] [CrossRef]
- Zeng, X.; Li, W.; Sun, H.; Luo, X.; Garg, S.; Liu, T.; Zhang, J.; Zhang, Y. Mental Health Outcomes in Perinatal Women During the Remission Phase of COVID-19 in China. Front. Psychiatry 2020, 11, 571876. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Liu, H.; Duan, C.; Li, C.; Fan, J.; Li, H.; Chen, L.; Xu, H.; Li, X.; et al. Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. Am. J. Obstet. Gynecol. 2020, 223, 240.e1–240.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Wu, T.; Shi, H.; Li, Q.; Jiang, H.; Zheng, D.; Wang, X.; Wei, Y.; Zhao, Y.; et al. Impact of COVID-19 in pregnancy on mother’s psychological status and infant’s neurobehavioral development: A longitudinal cohort study in China. BMC Med. 2020, 18, 347. [Google Scholar] [CrossRef]
- Kotabagi, P.; Nauta, M.; Fortune, L.; Yoong, W. COVID-19 positive mothers are not more anxious or depressed than non COVID pregnant women during the pandemic: A pilot case-control comparison. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, M.; Foulon, V.; Ngo, E.; Panchaud, A.; Winterfeld, U.; Pomar, L.; Lambelet, V.; Cleary, B.; O’Shaughnessy, F.; Passier, A.; et al. Mental health status of pregnant and breast-feeding women during the COVID-19 pandemic—A multinational cross-sectional study. Acta Obstet. Gynecol. Scand. 2021, 100, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hu, R.; Lu, C.; Huang, D.; Cui, D.; Huang, G.; Zhang, M. Investigation on the mental health status of pregnant women in China during the Pandemic of COVID-19. Arch. Gynecol. Obstet. 2020, 303, 463–469. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Llurba, E.; Figueras, F.; Gómez-Roig, M.D.; Gratacós, E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet 2020, 396, 530–531. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Borras, R.; Paules, C.; Casas, R.; Martín-Asuero, A.; Arranz, A.; Vieta, E.; Estruch, R.G.E. Mediterranean Diet, Mindfulness Based Stress Reduction and usual care during pregnancy for reducing fetal growth restriction and adverse perinatal outcomes: IMPACT BCN. A study protocol for a RCT. Trials 2021, 22, 362. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Bonnín, C.D.M.; Yatham, L.; Michalak, E.; Martínez-Arán, A.; Dhanoa, T.; Torres, I.; Santos-Pascual, C.; Valls, E.; Carvalho, A.; Sanchez-Moreno, J.; et al. Psychometric properties of the well-being index (WHO-5) spanish version in a sample of euthymic patients with bipolar disorder. J. Affect. Disord. 2017, 228, 153–159. [Google Scholar] [CrossRef]
- Linton, M.J.; Dieppe, P.; Medina-Lara, A. Review of 99 self-report measures for assessing well-being in adults: Exploring dimensions of well-being and developments over time. BMJ Open 2016, 6, e010641. [Google Scholar] [CrossRef]
- Mortazavi, F.; Mehrabad, M.; Kiaee Tabar, R. Pregnant Women’s Well-being and Worry during the COVID-19 Pandemic: A Comparative Study. BMC Pregnancy Childbirth 2021, 21, 59. [Google Scholar]
- Saccone, G.; Florio, A.; Aiello, F.; Venturella, R.; De Angelis, M.C.; Locci, M.; Bifulco, G.; Zullo, F.; Sardo, A.D.S. Psychological impact of coronavirus disease 2019 in pregnant women. Am. J. Obstet. Gynecol. 2020, 223, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, N.; Lemieux, R.; Garon-Bissonnette, J.; Drouin-Maziade, C.; Martel, É.; Maziade, M. Uptrend in distress and psychiatric symptomatology in pregnant women during the coronavirus disease 2019 pandemic. Acta Obstet. Gynecol. Scand. 2020, 99, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, V.; Manghina, V.; Giliberti, L.; Vettore, M.; Severino, L.; Straface, G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int. J. Gynecol. Obstet. 2020, 150, 184–188. [Google Scholar] [CrossRef]
- Perzow, S.E.; Hennessey, E.-M.P.; Hoffman, M.C.; Grote, N.K.; Davis, E.P.; Hankin, B.L. Mental health of pregnant and postpartum women in response to the COVID-19 pandemic. J. Affect. Disord. Rep. 2021, 4, 100123. [Google Scholar] [CrossRef]
- Ravaldi, C.; Ricca, V.; Wilson, A.; Homer, C.; Vannacci, A. Previous psychopathology predicted severe COVID-19 concern, anxi-ety and PTSD symptoms in pregnant women during lockdown in Italy. medRxiv 2020, 23, 783–786. [Google Scholar]
- Ceulemans, M.; Hompes, T.; Foulon, V. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic: A call for action. Int. J. Gynecol. Obstet. 2020, 151, 146–147. [Google Scholar] [CrossRef]
- Farrell, T.; Reagu, S.; Mohan, S.; Elmidany, R.; Qaddoura, F.; Ahmed, E.E.; Corbett, G.; Lindow, S.; Abuyaqoub, S.M.; Alabdulla, M.A. The impact of the COVID-19 pandemic on the peri-natal mental health of women. J. Perinat Med. 2020, 48, 971–976. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wang, Y.; Sun, L.; Zhang, J.; Shi, Y.; Wang, J.; Zhang, H.; Sun, G.; Baker, P.N.; et al. Prenatal anxiety and obstetric decisions among pregnant women in Wuhan and Chongqing during the COVID-19 outbreak: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1229–1240. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Ayaz, R.; Gunay, T.; Akin, E.; Turgut, A.; Karateke, A. Anxiety and post-traumatic stress disorder symptoms in pregnant women during the COVID-19 pandemic’s delay phase. Psychiatr. Danub. 2020, 32, 521–526. [Google Scholar] [CrossRef]
- Garcia-Basteiro, A.L.; Moncunill, G.; Tortajada, M.; Vidal, M.; Guinovart, C.; Jimenez, A.; Santano, R.; Sanz, S.; Méndez, S.; Llupià, A.; et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat. Commun. 2020, 11, 3500. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total Cohort (n = 1320) |
|---|---|
| Age (years) | 33.3 (29.1–37) |
| Ethnicity | |
| White | 858 (65%) |
| Latin American | 297 (22.5%) |
| Black | 23 (1.7%) |
| Asian | 81 (6.1%) |
| Others | 61 (4.6%) |
| Education level | |
| Not educated | 31 (2.3%) |
| Primary | 86 (6.5%) |
| Secondary | 361 (27.3%) |
| Vocational | 191 (14.5%) |
| University | 651 (49.3%) |
| Working status | |
| Employed | 930 (70.5%) |
| Unemployed | 262 (19.8) |
| Housewife | 113 (8.6%) |
| Student | 15 (1.1%) |
| Low socio-economic status | 417 (31.6%) |
| Tobacco use during pregnancy | 127 (9.6%) |
| Pre-pregnancy BMI (kg/h2) | 24.1 (4.7) |
| Medical history | |
| Obesity (BMI > 30) | 157 (11.9%) |
| Psychiatric disorders * | 28 (2.1%) |
| Cardiac diseases | 45 (3.4%) |
| Respiratory disorders | 65 (4.9%) |
| Diabetes Mellitus | 18 (1.4%) |
| Thyroid diseases | 91 (6.9%) |
| Obstetric history | |
| Nulliparous | 724 (54.9%) |
| Assisted reproductive technologies | 98 (7.4%) |
| Characteristics | Total Cohort (n = 1320) |
|---|---|
| Preeclampsia | 57 (4.3%) |
| Threatened/spontaneous preterm delivery | 55 (4.2%) |
| Preterm premature rupture of the membranes | 40 (3%) |
| Stillbirth | 7 (0.5%) |
| Induction of labor | 509 (38.6%) |
| Gestational age at recruitment | |
| In first trimester | 10.7 (9.9–12.1) |
| In third trimester | 39.7 (38.6–40.6) |
| Gestational age at delivery | 39.2 (2.2) |
| Prematurity (<37 weeks) | 84 (6.4%) |
| Mode of delivery | |
| Vaginal delivery | 851 (64.5%) |
| Operative vaginal delivery | 123 (9.3%) |
| Cesarean section | 346 (26.2%) |
| Fetal distress | 123 (9.3%) |
| Female gender | 616 (46.7%) |
| Birth weight (grams) | 3280 (2985–3580) |
| Birth weight percentile | 48 (24–74) |
| Small for gestational age (<10th centile) | 154 (11.7%) |
| Severe small for gestational age (<3rd centile) | 52 (3.9%) |
| Large for gestational age (>90th centile) | 157 (11.9%) |
| 5-min Apgar 5 score | 9.9 (0.7) |
| Neonatal complications | 52 (3.9%) |
| Characteristics | WHO-5 ≤ 52 (n = 565) | WHO-5 > 52 (n = 755) | p-Value |
|---|---|---|---|
| Age (years) | 32.8 (28.8–37) | 33.6 (29.6–37.2) | 0.050 |
| Ethnicity | |||
| White | 367 (65%) | 491 (65%) | 0.977 |
| Latin American | 135 (23.9%) | 162 (21.5%) | 0.294 |
| Black | 6 (1.1%) | 17 (2.3%) | 0.102 |
| Asian | 37 (6.5%) | 44 (5.8%) | 0.589 |
| Others | 20 (3.5%) | 41 (5.4%) | 0.105 |
| Education level | |||
| Not educated | 13 (2.3%) | 18 (2.4%) | 0.921 |
| Primary | 35 (6.2%) | 51 (6.8%) | 0.683 |
| Secondary | 168 (29.7%) | 192 (25.6%) | 0.092 |
| Vocational | 76 (13.5%) | 115 (15.2%) | 0.363 |
| University | 273 (48.3%) | 378 (50.1%) | 0.530 |
| Working status | |||
| Employed | 396 (70.1%) | 534 (70.7%) | 0.801 |
| Unemployed | 107 (19%) | 154 (20.4%) | 0.520 |
| Housewife | 54 (9.6%) | 59 (7.8%) | 0.259 |
| Student | 7 (1.2%) | 8 (1.1%) | 0.761 |
| Low socio-economic status | 182 (32.2%) | 235 (31.1%) | 0.674 |
| Tobacco use during pregnancy | 53 (9.4%) | 74 (9.8%) | 0.798 |
| BMI (kg/h2) | 24 (4.6) | 24.2 (4.8) | 0.340 |
| Medical history | |||
| Obesity (BMI > 30) | 67 (11.9%) | 90 (11.9%) | 0.972 |
| Psychiatric disorders * | 23 (4.1%) | 5 (0.7%) | <0.001 |
| Cardiac diseases | 13 (2.3%) | 32 (4.2%) | 0.055 |
| Respiratory disorders | 29 (5.1%) | 36 (4.8%) | 0.762 |
| Diabetes Mellitus | 6 (1.1%) | 12 (1.6%) | 0.414 |
| Thyroid diseases | 30 (5.3%) | 61 (8.1%) | 0.049 |
| Obstetric history | |||
| Nulliparous | 314 (55.6%) | 411 (54.4%) | 0.681 |
| Assisted reproductive technologies | 36 (6.4%) | 62 (8.2%) | 0.207 |
| Characteristics | WHO-5 ≤ 52 (n = 565) | WHO-5 > 52 (n = 755) | p-Value |
|---|---|---|---|
| Trimester | <0.001 | ||
| First trimester | 117 (20.7%) | 327 (43.3%) | |
| Third trimester | 448 (79.3%) | 428 (56.7%) | |
| Preeclampsia | 28 (5%) | 29 (3.8%) | 0.324 |
| Threatened/spontaneous preterm labor | 29 (5.2%) | 25 (3.6%) | 0.147 |
| Preterm premature rupture of the membranes | 15 (2.7%) | 25 (3.3%) | 0.491 |
| Stillbirth | 3 (0.5%) | 4 (0.5%) | 0.998 |
| Induction of labor | 226 (40%) | 283 (37.5%) | 0.353 |
| Gestational age at delivery | 39.1 (2.3) | 39.3 (2.1) | 0.316 |
| Prematurity (<37 weeks) | 40 (7.1%) | 44 (5.8%) | 0.357 |
| Mode of delivery | |||
| Vaginal delivery | 361 (63.9%) | 490 (64.9%) | 0.705 |
| Operative vaginal delivery | 56 (9.9%) | 67 (8.9%) | 0.551 |
| Cesarean section | 148 (26.2%) | 198 (26.2%) | 0.990 |
| Fetal distress | 61 (10.8%) | 62 (8.2%) | 0.110 |
| Female gender | 269 (47.6%) | 347 (46%) | 0.552 |
| Birth weight (grams) | 3260 (2940–3560) | 3295 (3020–3595) | 0.076 |
| Birth weight percentile | 45 (21–74) | 50 (27–74) | 0.47 |
| Small for gestational age (<10th centile) | 67 (11.9%) | 87 (11.5%) | 0.851 |
| Severe small for gestational age (<3rd centile) | 22 (3.9%) | 30 (4%) | 0.941 |
| Large for gestational age (>90th centile) | 68 (12%) | 89 (11.8%) | 0.891 |
| 5-min Apgar score | 9.8 (0.8) | 9.9 (0.7) | 0.268 |
| Neonatal complications | 29 (5.1%) | 23 (3%) | 0.054 |
| Characteristics | WHO-5 ≤ 52 (n = 565) | WHO-5 > 52 (n = 755) | p-Value |
|---|---|---|---|
| Positive SARS-CoV-2 testing | 88 (15.6%) | 114 (15.1%) | 0.812 |
| Symptoms of SARS-CoV-2 infection within the last 10 weeks | 95 (16.8%) | 87 (11.5%) | 0.006 |
| Fever | 25 (4.4%) | 19 (2.5%) | 0.056 |
| Dry cough | 44 (7.8%) | 31 (4.1%) | 0.004 |
| Difficulty breathing or shortness of breath | 17 (3%) | 12 (1.6%) | 0.082 |
| Diarrhea | 20 (3.5%) | 16 (2.1%) | 0.117 |
| Other respiratory symptoms | 9 (1.6%) | 8 (1.2%) | 0.534 |
| Myalgia | 17 (3%) | 17 (2.3%) | 0.390 |
| Skin rash | 5 (0.9%) | 4 (0.5%) | 0.438 |
| Loss of taste or smell | 15 (2.7%) | 12 (1.6%) | 0.176 |
| Other | 10 (1.8%) | 16 (2.1%) | 0.651 |
| Combination of symptoms predictable for SARS-CoV-2 infection | |||
| At least two symptoms or anosmia | 44 (7.8%) | 39 (5.2%) | 0.052 |
| At least three symptoms or anosmia | 22 (3.9%) | 20 (2.6%) | 0.202 |
| Fever, cough and dyspnea | 8 (1.4%) | 1 (0.1%) | 0.005 |
| Symptom-relatedCOVID-19 severity | |||
| Mild | 2 (14.5%) | 79 (10.5%) | 0.026 |
| Moderate | 5 (0.9%) | 7 (0.9%) | 0.936 |
| Severe | 8 (1.4%) | 1 (0.1%) | 0.005 |
| COVID-19 disease | |||
| Hospital admission for COVID-19 disease | 15 (2.7%) | 3 (0.4%) | <0.001 |
| Pneumonia | 3 (0.5%) | 1 (0.1%) | 0.192 |
| Severe pneumonia | 2 (0.4%) | 1 (0.1%) | 0.403 |
| Oxygen support | 2 (0.4%) | 1 (0.1%) | 0.403 |
| Admission to intensive care unit | 1 (0.2%) | 1 (0.1%) | 0.837 |
| Invasive ventilatory support | 1 (0.2%) | 0 (0%) | 0.248 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-Value | Betta Coefficient | |
| Baseline maternal characteristics | |||||
| Age (years) | 0.98 (0.96–1) | 0.051 | |||
| Gestational age at recruitment (weeks) | 1.04 (1.03–1.05) | <0.001 | |||
| Non-European ethnicity | 1 (0.8–1.3) | 0.977 | |||
| Low socio-economic status | 1 (0.8–1.3) | 0.674 | |||
| Tobacco use during pregnancy | 0.95 (0.7–1.4) | 0.789 | |||
| Psychiatric disorders | 6.4 (2.4–16.9) | <0.001 | 7.1 (2.6–19) | <0.001 | 1.947 |
| Thyroid diseases | 0.6 (0.4–1) | 0.051 | |||
| Nulliparity | 1 (0.8–1.3) | 0.681 | |||
| Assisted reproductive techniques | 0.7 (0.5–1.2) | 0.208 | |||
| Pregnancy outcomes | |||||
| Trimester (first vs. third) | 1.7 (1.5–1.9) | <0.001 | 1.7 (1.5–2) | <0.001 | 0.537 |
| Induction of labor | 1.1 (0.9–1.4) | 0.353 | |||
| Cesarean section | 0.99 (0.8–1.3) | 0.99 | |||
| SARS-CoV-2 status | |||||
| Positive SARS-CoV-2 testing | 1 (0.8–1.4) | 0.812 | |||
| Presence of at least one COVID-19 symptom | 1.5 (1.1–2.1) | 0.006 | |||
| Presence of fever, cough and dyspnea | 10.8 (1.3–86.8) | 0.025 | |||
| Presence of severe COVID-19 symptoms | 10.8 (1.3–86.8) | 0.025 | |||
| Hospital admission for COVID-19 | 6.8 (1.9–23.7) | 0.002 | 4.8 (1.4–16.7) | 0.014 | 1.565 |
| Constant | −1.606 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascal, R.; Crovetto, F.; Casas, I.; Youssef, L.; Trilla, C.; Larroya, M.; Cahuana, A.; Boada, D.; Foraster, M.; Llurba, E.; et al. Impact of the COVID-19 Pandemic on Maternal Well-Being during Pregnancy. J. Clin. Med. 2022, 11, 2212. https://doi.org/10.3390/jcm11082212
Pascal R, Crovetto F, Casas I, Youssef L, Trilla C, Larroya M, Cahuana A, Boada D, Foraster M, Llurba E, et al. Impact of the COVID-19 Pandemic on Maternal Well-Being during Pregnancy. Journal of Clinical Medicine. 2022; 11(8):2212. https://doi.org/10.3390/jcm11082212
Chicago/Turabian StylePascal, Rosalia, Francesca Crovetto, Irene Casas, Lina Youssef, Cristina Trilla, Marta Larroya, Alex Cahuana, David Boada, Maria Foraster, Elisa Llurba, and et al. 2022. "Impact of the COVID-19 Pandemic on Maternal Well-Being during Pregnancy" Journal of Clinical Medicine 11, no. 8: 2212. https://doi.org/10.3390/jcm11082212
APA StylePascal, R., Crovetto, F., Casas, I., Youssef, L., Trilla, C., Larroya, M., Cahuana, A., Boada, D., Foraster, M., Llurba, E., Sunyer, J., Crispi, F., Gratacos, E., & Gómez-Roig, M. D. (2022). Impact of the COVID-19 Pandemic on Maternal Well-Being during Pregnancy. Journal of Clinical Medicine, 11(8), 2212. https://doi.org/10.3390/jcm11082212







