Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Patients
2.3. Statistical Analysis
3. Results
3.1. Sample Description
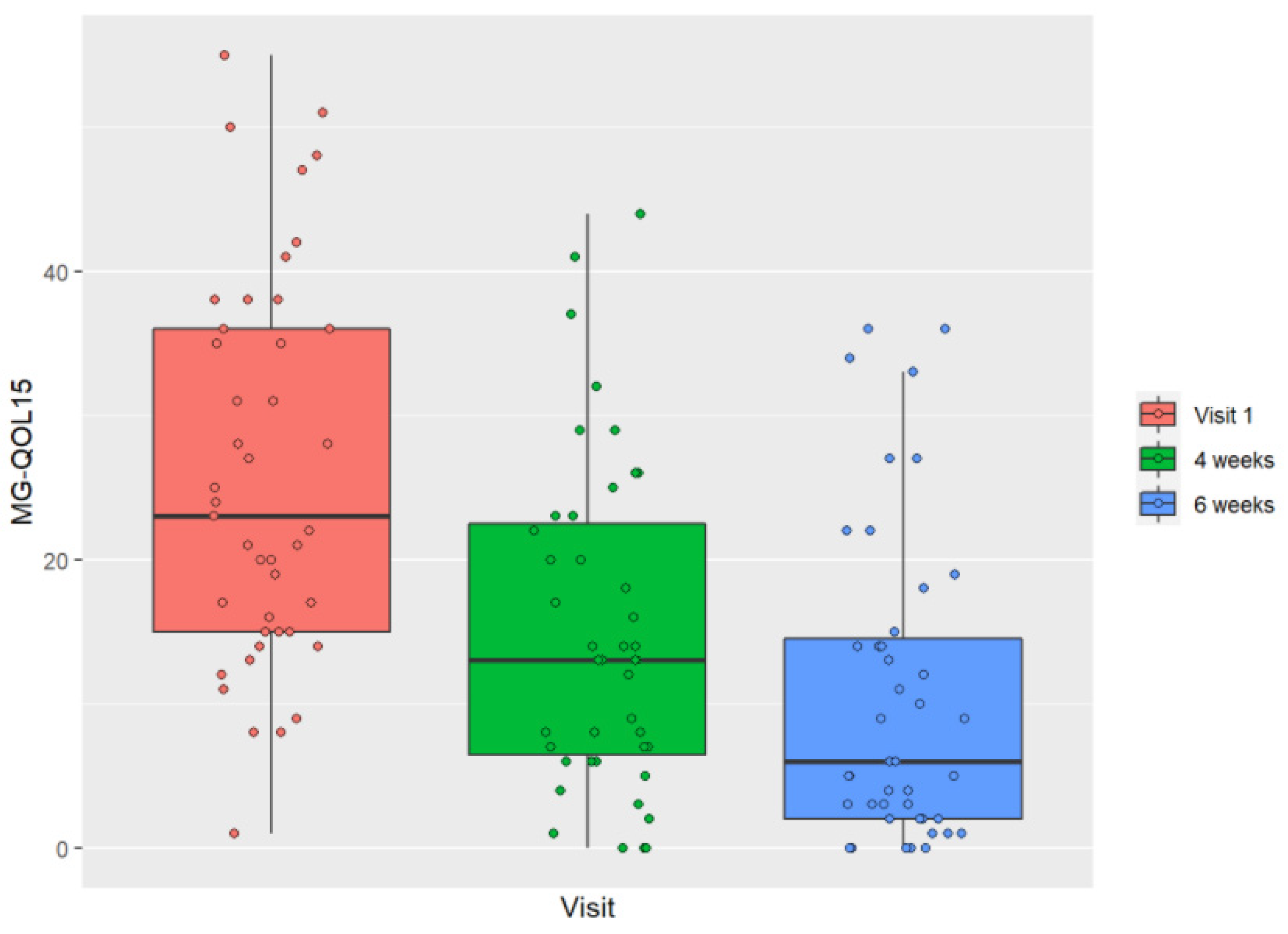
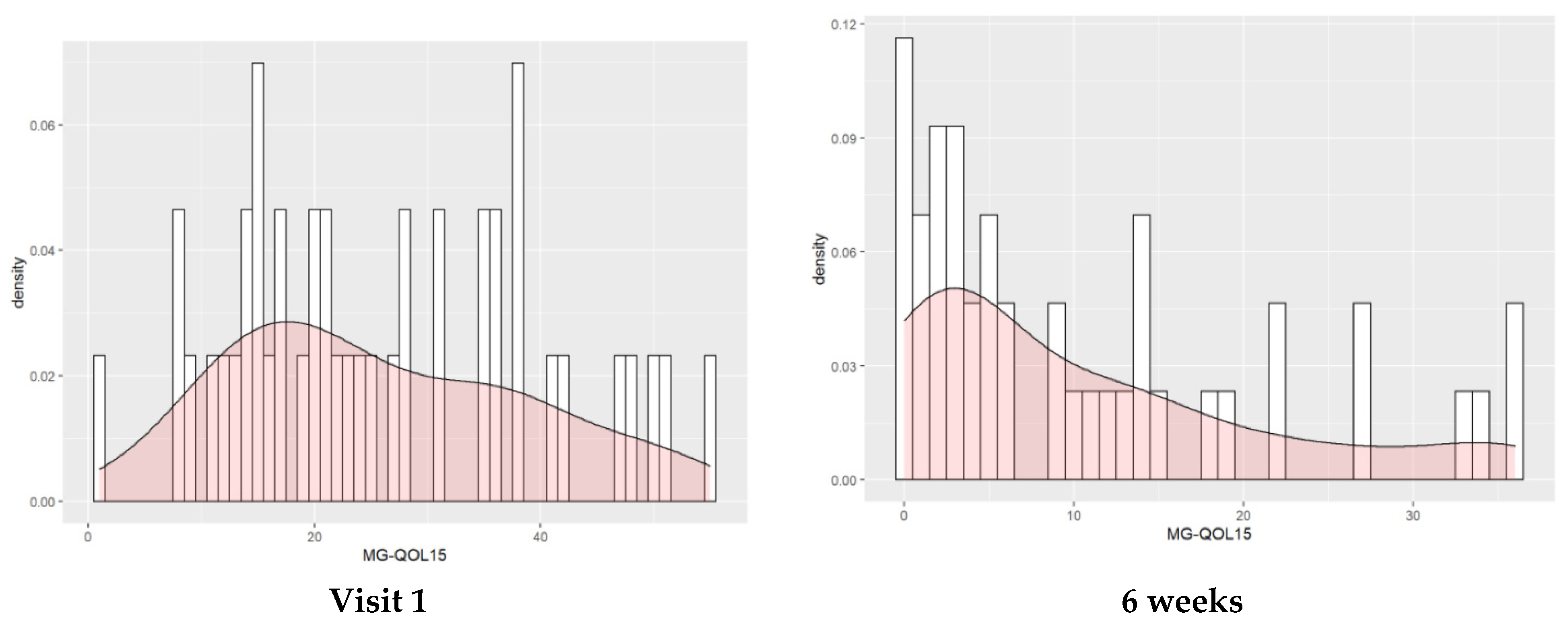
3.2. Evolution of HRQOL throughout the Study
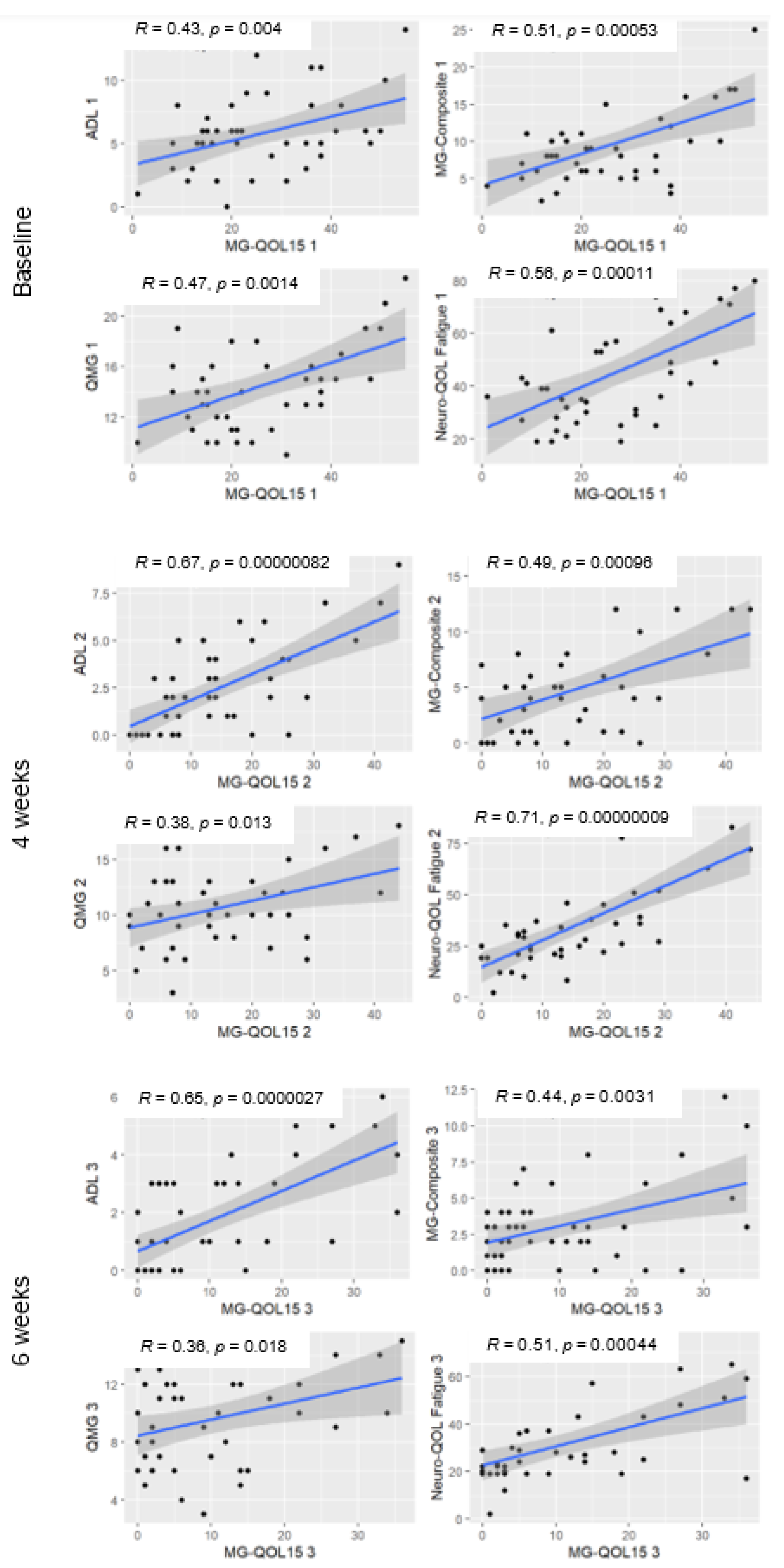
3.3. Correlation of the MG-QOL15 with Other Functional Scales
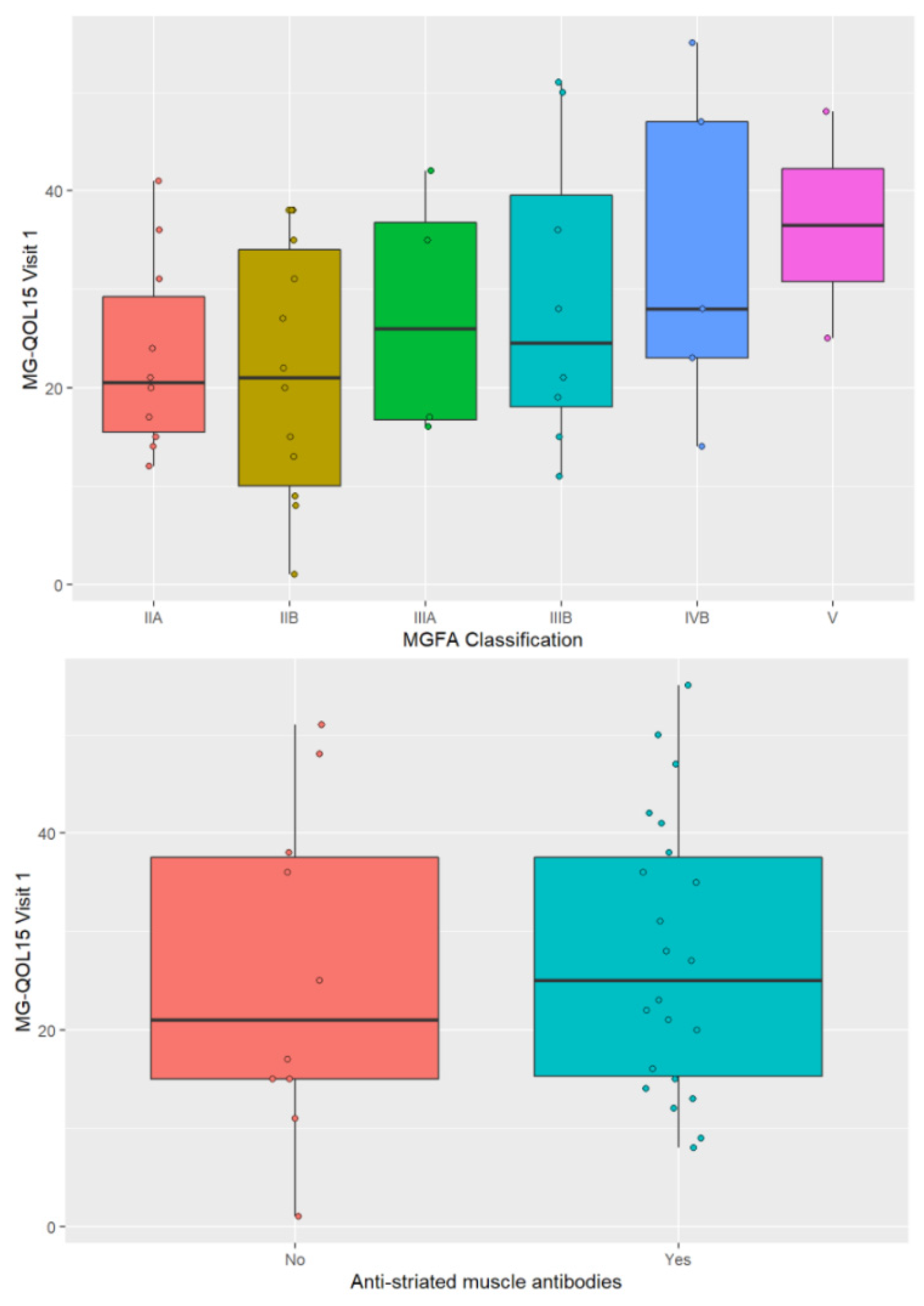
3.4. Factors Related to HRQOL
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Díez-Porras, L.; Homedes, C.; Alberti, M.A.; Vélez-Santamaría, V.; Casasnovas, C. Intravenous immunoglobulins may prevent prednisone-exacerbation in myasthenia gravis. Sci. Rep. 2020, 10, 13497. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, T.M.; Conaway, M.R.; Cutter, G.R.; Sanders, D.B.; McDermott, M.; Thornton, C.; Tawil, R.; Barohn, R.J.; Group, M.S. Less is more, or almost as much: A 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve 2008, 38, 957–963. [Google Scholar] [CrossRef]
- Burns, T.M.; Grouse, C.K.; Conaway, M.R.; Sanders, D.B.; Solorzano, G.; Farrugia, M.E.; Massey, J.M.; Juel, V.C.; Hobson-Webb, L.D.; Tucker-Lipscomb, B.; et al. Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve 2010, 41, 219–226. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Guo, J.; Ma, S.; Fan, L.; Wang, X.; Li, C.; Guo, P.; Wang, J.; Li, H.; et al. Quality of life in 188 patients with myasthenia gravis in China. Int. J. Neurosci. 2016, 126, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.N.; Xin, H.; L’Italien, G.J.; Harris, L.A.; Cutter, G.R. Impact of Refractory Myasthenia Gravis on Health-Related Quality of Life. J. Clin. Neuromuscul. Dis. 2019, 20, 173–181. [Google Scholar] [CrossRef]
- Boldingh, M.I.; Dekker, L.; Maniaol, A.H.; Brunborg, C.; Lipka, A.F.; Niks, E.H.; Verschuuren, J.J.G.M.; Tallaksen, C.M.E. An up-date on health-related quality of life in myasthenia gravis -results from population based cohorts. Health Qual. Life Outcomes 2015, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- Alanazy, M.H.; Binabbad, R.S.; Alromaih, N.I.; Almansour, R.A.; Alanazi, S.N.; Alhamdi, M.F.; Alazwary, N.; Muayqil, T. Severity and depression can impact quality of life in patients with myasthenia gravis. Muscle Nerve 2020, 61, 69–73. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Herbelin, L.; Nations, S.P.; Foster, B.; Bryan, W.W.; Barohn, R.J. Myasthenia gravis activities of daily living profile. Neurology 1999, 52, 1487–1489. [Google Scholar] [CrossRef]
- Muppidi, S. The Myasthenia Gravis-Specific Activities of Daily Living Profile. Ann. N. Y. Acad. Sci. 2012, 1274, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.M.; Conaway, M.; Sanders, D.B. The MG Composite: A valid and reliable outcome measure for myasthenia gravis. Neurology 2010, 74, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barohn, R.J.; McIntire, D.; Herbelin, L.; Wolfe, G.I.; Nations, S.; Bryan, W.W. Reliability Testing of the Quantitative Myasthenia Gravis Score. Ann. N. Y. Acad. Sci. 1998, 841, 769–772. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders (NINDS). Available online: https://www.ninds.nih.gov/ (accessed on 3 March 2022).
- Burns, T.M.; Grouse, C.K.; Wolfe, G.I.; Conaway, M.R.; Sanders, D.B.; Group, M.C. and M.-O.S. The MG-QOL15 for following the health-related quality of life of patients with myasthenia gravis. Muscle Nerve 2011, 43, 14–18. [Google Scholar] [CrossRef]
- McPherson, T.; Aban, I.; Duda, P.W.; Farzaneh-Far, R.; Wolfe, G.I.; Kaminski, H.J.; Cutter, G.; Lee, I. Correlation of Quantitative Myasthenia Gravis and Myasthenia Gravis Activities of Daily Living scales in the MGTX study. Muscle Nerve 2020, 62, 261–266. [Google Scholar] [CrossRef]
- Utsugisawa, K.; Suzuki, S.; Nagane, Y.; Masuda, M.; Murai, H.; Imai, T.; Tsuda, E.; Konno, S.; Nakane, S.; Suzuki, Y.; et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve 2014, 50, 493–500. [Google Scholar] [CrossRef]
- Mansueto, A.M.; Gomez, R.S.; Mageste Barbosa, L.S.; Freitas, D.D.S.; Comini-Frota, E.R.; Kummer, A.; Aguiar Lemos, S.M.; Teixeira, A.L. Determinants of quality of life in Brazilian patients with myasthenia gravis. Clinics 2016, 71, 370–374. [Google Scholar] [CrossRef]
- Kumar, R.; Nagappa, M.; Sinha, S.; Taly, A.B.; Rao, S. MG-QoL-15 scores in treated myasthenia gravis: Experience from a university hospital in India. Neurol. India 2016, 64, 405–410. [Google Scholar]
- Rostedt, A.; Padua, L.; Stålberg, E.V. Correlation between regional myasthenic weakness and mental aspects of quality of life. Eur. J. Neurol. 2006, 13, 191–193. [Google Scholar] [CrossRef]
- Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Ann. Thorac. Surg. 2000, 70, 327–334. [Google Scholar] [CrossRef]
- Padua, L.; Evoli, A.; Aprile, I.; Caliandro, P.; Mazza, S.; Padua, R.; Tonali, P. Health-related quality of life in patients with myasthemia gravis and the relationship between patient-oriented assessment and conventional measurements. Neurol. Sci. 2001, 22, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mullins, L.L.; Carpentier, M.Y.; Paul, R.H.; Sanders, D.B.; McDermott, M.; Thornton, C.; Tawil, R.; Barohn, R.J.; Group, M.S. Disease-specific measure of quality of life for myasthenia gravis. Muscle Nerve 2008, 38, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Basta, I.Z.; Pekmezović, T.D.; Perić, S.Z.; Kisić-Tepavčević, D.B.; Rakočević-Stojanović, V.M.; Stević, Z.D.; Lavrnic, D.V. Assessment of health-related quality of life in patients with myasthenia gravis in Belgrade (Serbia). Neurol. Sci. 2012, 33, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Romi, F.; Hong, Y.; Gilhus, N.E. Pathophysiology and immunological profile of myasthenia gravis and its subgroups. Curr. Opin. Immunol. 2017, 49, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; López De Munain, A.; et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Raggi, A.; Leonardi, M.; Mantegazza, R.; Casale, S.; Fioravanti, G. Social support and self-efficacy in patients with Myasthenia Gravis: A common pathway towards positive health outcomes. Neurol. Sci. 2010, 31, 231–235. [Google Scholar] [CrossRef]
- Twork, S.; Wiesmeth, S.; Klewer, J.; Pöhlau, D.; Kugler, J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual. Life Outcomes 2010, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Barnett, C.; Wilson, G.; Barth, D.; Katzberg, H.D.; Bril, V. Changes in quality of life scores with intravenous immunoglobulin or plasmapheresis in patients with myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 94–97. [Google Scholar] [CrossRef]
- Howard, J.F.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986. [Google Scholar] [CrossRef]
- Muppidi, S.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve 2019, 60, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Kaminski, H.J.; Xin, H.; Cutter, G. Gender and quality of life in myasthenia gravis patients from the myasthenia gravis foundation of America registry. Muscle Nerve 2018, 58, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Mantegazza, R.; Wang, J.J.; O’Brien, F.; Patra, K.; Howard, J.F.; Mazia, C.G.; Wilken, M.; Barroso, F.; Saba, J.; et al. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual. Life Res. 2019, 28, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, T.M.; Sadjadi, R.; Utsugisawa, K.; Gwathmey, K.G.; Joshi, A.; Jones, S.; Bril, V.; Barnett, C.; Guptill, J.T.; Sanders, D.B.; et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve 2016, 54, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
| Total (N) | ||
|---|---|---|
| Sex | 45 | |
| Woman, n (%) | 15 (33.33) | |
| Man, n (%) | 30 (66.67) | |
| Age | ||
| Minimum | 26 | |
| Maximum | 85 | |
| Median | 69 | |
| Age at onset, mean (standard deviation) | 62.22 (16.32) | |
| MGFA class | ||
| I, n (%) | 0 (0) | |
| IIA, n (%) | 10 (22.2) | |
| IIB, n (%) | 14 (31.1) | |
| IIIA, n (%) | 5 (11.1) | |
| IIIB, n (%) | 9 (20) | |
| IV, n (%) | 5 (11.1) | |
| V, n (%) | 2 (4.4) | |
| Antibodies | ||
| Anti-RAch, n (%) | 41 (91.1) | |
| 0 (0) | ||
| Anti-MuSK, n (%) | 4 (8.9) | |
| Double seronegative, n (%) | 22 (48.9) | |
| Anti-striated muscle, n (%) | ||
| Thymus | ||
| Thymoma, n (%) | 2 (4.4) | |
| Thymic hyperplasia, n (%) | 4 (8.9) | |
| Atrophy or CT without evidence of thymoma, n (%) | 39 (86.7) | |
| Score on scales (pretreatment and at V1) | ||
| Pretreatment QMG, Mean (standard deviation) | 17.04 (3.83) | 25 |
| Pretreatment QMG, Median [25%, 75%] | 17 [14; 20] | 25 |
| V1 MG-QOL, Mean (standard deviation) | 25.93 (13.42) | 43 |
| V1 MG-QOL, Median [25%; 75%] | 23 [15; 36] | 43 |
| V1 ADL, Mean (standard deviation) | 5.84 (2.92) | 45 |
| V1 ADL, Median [25%; 75%] | 6 [4; 7] | 45 |
| V1 QMG, Mean (standard deviation) | 14.4 (3.63) | 45 |
| V1 QMG, Median [25%; 75%] | 14 [11; 16] | 45 |
| V1 MG-Composite, Mean (standard deviation) | 9.6 (5.44) | 45 |
| V1 MG-Composite, Median [25%; 75%] | 8 [6; 11] | 45 |
| V1 Neuro-QoL fatigue, Mean (standard deviation) | 44.44 (19.31) | 43 |
| V1 Neuro-QoL fatigue, Median [25%; 75%] | 39 [28.5; 59] | 43 |
| Visit 1 | 4 Weeks | 6 Weeks | 4 Weeks–Visit 1 | 6 Weeks–Visit 1 | 6 Weeks–4 Weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CI | Mean | CI | Mean | CI | Dif | CI | Pval | Dif | CI | Pval | Dif | CI | Pval | |
| MG-QOL15 | 25.93 | [22.32, 29.54] | 14.91 | [11.3, 18.52] | 10.53 | [6.93, 14.14] | −11.02 | [−15.05, −7] | <0.0001 | −15.4 | [−19.42, −11.37] | <0.0001 | −4.37 | [−8.4, −0.34] | 0.0301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez Porras, L.; Homedes, C.; Alberti, M.A.; Velez Santamaria, V.; Casasnovas, C. Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales. J. Clin. Med. 2022, 11, 2189. https://doi.org/10.3390/jcm11082189
Diez Porras L, Homedes C, Alberti MA, Velez Santamaria V, Casasnovas C. Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales. Journal of Clinical Medicine. 2022; 11(8):2189. https://doi.org/10.3390/jcm11082189
Chicago/Turabian StyleDiez Porras, Laura, Christian Homedes, Maria Antonia Alberti, Valentina Velez Santamaria, and Carlos Casasnovas. 2022. "Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales" Journal of Clinical Medicine 11, no. 8: 2189. https://doi.org/10.3390/jcm11082189
APA StyleDiez Porras, L., Homedes, C., Alberti, M. A., Velez Santamaria, V., & Casasnovas, C. (2022). Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales. Journal of Clinical Medicine, 11(8), 2189. https://doi.org/10.3390/jcm11082189






