Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival
Abstract
:1. Introduction
2. Materials and Methods
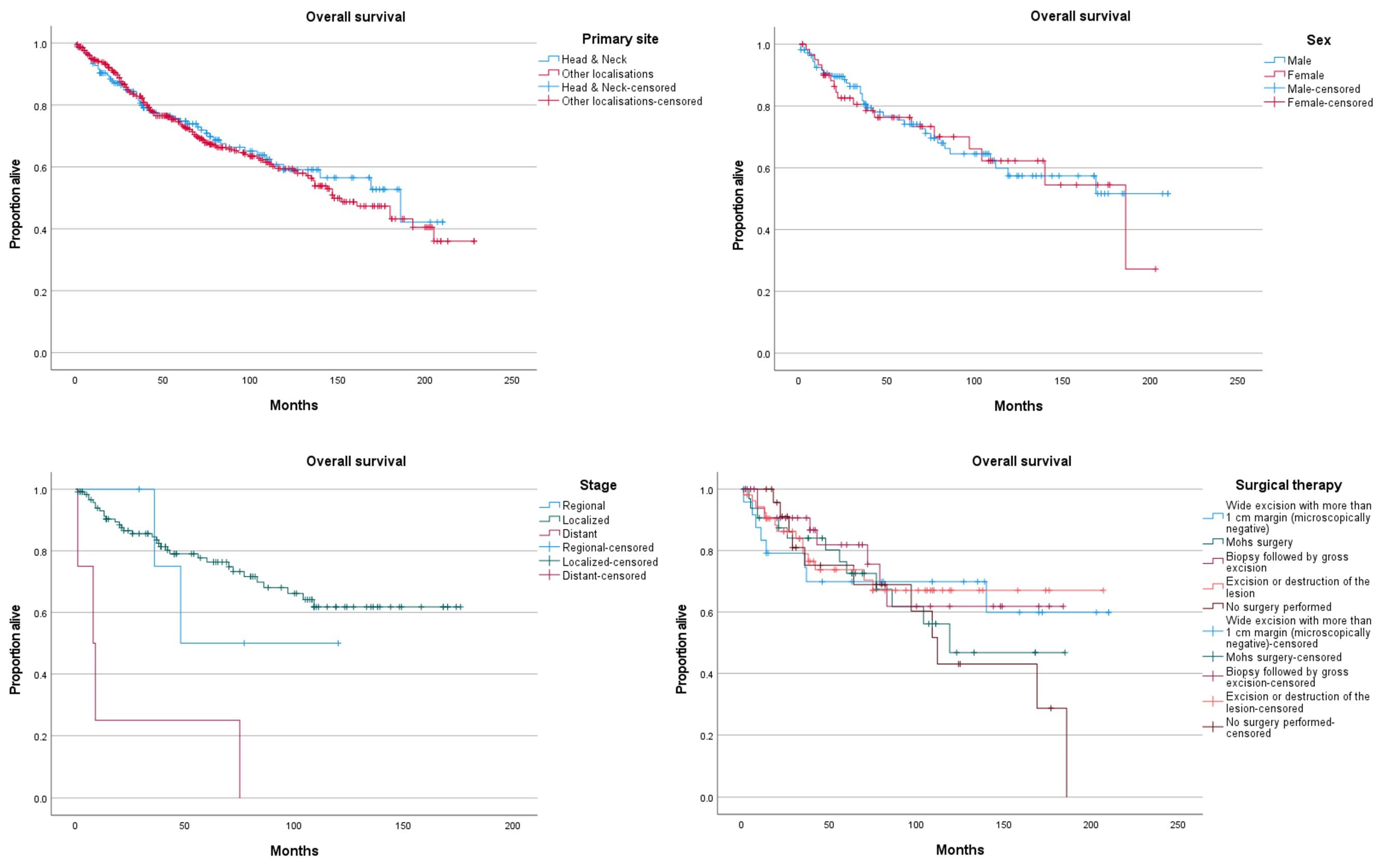
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goon, P.; Gurung, P.; Levell, N.; Subramanian, P.; Yong, A.; Lee, K.; Igali, L.; Greenberg, D.; Shah, S.; Tan, E. Eccrine Porocarcinoma of the Skin is Rising in Incidence in the East of England. Acta Derm. Venereol. 2018, 98, 991–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, R.; Ezaldein, H.H.; Scott, J.F.; Bordeaux, J.S. Trends in the incidence and survival of eccrine malignancies in the United States: A SEER population-based study. J. Am. Acad. Dermatol. 2019, 80, 1769–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Giorgi, V.; Salvati, L.; Barchielli, A.; Caldarella, A.; Gori, A.; Scarfì, F.; Savarese, I.; Pimpinelli, N.; Urso, C.; Massi, D. The burden of cutaneous adnexal carcinomas and the risk of associated squamous cell carcinoma: A population-based study. Br. J. Dermatol. 2019, 180, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, A.; Pukkala, E.; Böhling, T.; Koljonen, V. Malignant Eccrine Porocarcinoma in Finland During 2007 to 2017. Acta Derm. Venereol. 2021, 101, adv00363. [Google Scholar] [CrossRef] [PubMed]
- Pinkus, H.; Mehregan, A.H. Epidermotropic Eccrine Carcinoma: A Case Combining Features of Eccrine Poroma and Paget’s Dermatosis. Arch. Dermatol. 1963, 88, 597–606. [Google Scholar] [CrossRef]
- Joshy, J.; Mistry, K.; Levell, N.J.; van Bodegraven, B.; Vernon, S.; Rajan, N.; Craig, P.; Venables, Z.C. Porocarcinoma—A review. Clin. Exp. Dermatol. 2022. [Google Scholar] [CrossRef]
- Riera-Leal, L.; Guevara-Gutiérrez, E.; Barrientos-García, J.G.; Madrigal-Kasem, R.; Briseño-Rodríguez, G.; Tlacuilo-Parra, A. Eccrine porocarcinoma: Epidemiologic and histopathologic characteristics. Int. J. Dermatol. 2015, 54, 580–586. [Google Scholar] [CrossRef]
- Urso, C.; Bondi, R.; Paglierani, B.M.; Salvadori, A.; Anichini, C.; Giannini, A. Carcinomas of Sweat Glands: Report of 60 cases. Arch. Pathol. Lab. Med. 2001, 125, 498–505. [Google Scholar] [CrossRef]
- Le, N.; Janik, S.; Liu, D.T.; Grasl, S.; Faisal, M.; Pammer, J.; Schickinger-Fischer, B.; Hamzavi, J.; Seemann, R.; Erovic, B.M. Eccrine porocarcinoma of the head and neck: Meta-analysis of 120 cases. Head Neck 2020, 42, 2644–2659. [Google Scholar] [CrossRef]
- Nazemi, A.; Higgins, S.; Swift, R.; In, G.; Miller, K.; Wysong, A. Eccrine Porocarcinoma: New Insights and a Systematic Review of the Literature. Dermatol. Surg. 2018, 44, 1247–1261. [Google Scholar] [CrossRef]
- Avraham, J.B.; Villines, D.; Maker, V.K.; August, C.; Maker, A.V. Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: Risk factors and trends in outcomes. J. Surg. Oncol. 2013, 108, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.; Greene, J.; Ansari, N.; Kim, B.; Seed, P.T.; McKee, P.H.; Calonje, E. Eccrine Porocarcinoma (Malignant Eccrine Poroma): A Clinicopathologic Study of 69 Cases. Am. J. Surg. Pathol. 2001, 25, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Kakamad, F.; Baba, H.O.; Salih, R.Q.; Hawbash, M.; Mohammed, S.H.; Othman, S.; Saeed, Y.A.; Habibullah, I.J.; Muhialdeen, A.S.; et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann. Med. Surg. 2017, 20, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Marghitu, T.; Goyal, N.; Rubin, N.; Patel, K.; Goyal, K.; O’leary, D.; Bohjanen, K.; Maher, I. Surgical management and lymph-node biopsy of rare malignant cutaneous adnexal carcinomas: A population-based analysis of 7591 patients. Arch. Dermatol. Res. 2021, 313, 623–632. [Google Scholar] [CrossRef]
- Summary Staging Manual-2018. Available online: https://seer.cancer.gov/tools/ssm/index.html (accessed on 9 January 2022).
- Sawaya, J.L.; Khachemoune, A. Poroma: A review of eccrine, apocrine, and malignant forms. Int. J. Dermatol. 2014, 53, 1053–1061. [Google Scholar] [CrossRef]
- Sargen, M.R.; Cahoon, E.K.; Yu, K.J.; Madeleine, M.M.; Zeng, Y.; Rees, J.R.; Lynch, C.F.; Engels, E.A. Spectrum of Nonkeratinocyte Skin Cancer Risk Among Solid Organ Transplant Recipients in the US. JAMA Dermatol. 2022. [Google Scholar] [CrossRef]
- Mahomed, F.; Blok, J.; Grayson, W. The squamous variant of eccrine porocarcinoma: A clinicopathological study of 21 cases. J. Clin. Pathol. 2008, 61, 361–365. [Google Scholar] [CrossRef]
- Wain, R.; Tehrani, H. Reconstructive outcomes of Mohs surgery compared with conventional excision: A 13-month prospective study. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 946–952. [Google Scholar] [CrossRef]
- Xu, Y.G.; Aylward, J.; Longley, B.J.; Hinshaw, M.A.; Snow, S.N. Eccrine Porocarcinoma Treated by Mohs Micrographic Surgery: Over 6-Year Follow-up of 12 Cases and Literature Review. Dermatol. Surg. 2015, 41, 685–692. [Google Scholar] [CrossRef]
- Wittenberg, G.P.; Robertson, D.B.; Solomon, A.R.; Washington, C.V. Eccrine Porocarcinoma Treated with Mohs Micrographic Surgery: A Report of Five Cases. Dermatol. Surg. 1999, 25, 911–913. [Google Scholar] [CrossRef]
- Tolkachjov, S.N.; Hocker, T.L.; Camilleri, M.J.; Baum, C.L. Treatment of Porocarcinoma with Mohs Micrographic Surgery: The Mayo Clinic Experience. Dermatol. Surg. 2016, 42, 745–750. [Google Scholar] [CrossRef]
- Tsunoda, K.; Onishi, M.; Maeda, F.; Akasaka, T.; Sugai, T.; Amano, H. Evaluation of Sentinel Lymph Node Biopsy for Eccrine Porocarcinoma. Acta Derm. Venereol. 2019, 99, 691–692. [Google Scholar] [CrossRef] [Green Version]
- Storino, A.; Drews, R.E.; Tawa, N.E. Malignant Cutaneous Adnexal Tumors and Role of SLNB. J. Am. Coll. Surg. 2021, 232, 889–898. [Google Scholar] [CrossRef]
- De Iuliis, F.; Amoroso, L.; Taglieri, L.; Vendittozzi, S.; Blasi, L.; Salerno, G.; Lanza, R.; Scarpa, S. Chemotherapy of rare skin adnexal tumors: A review of literature. Anticancer Res. 2014, 34, 5263–5268. [Google Scholar]
- Wang, L.S.; Handorf, E.A.; Wu, H.; Liu, J.; Perlis, C.S.; Galloway, T.J. Surgery and Adjuvant Radiation for High-risk Skin Adnexal Carcinoma of the Head and Neck. Am. J. Clin. Oncol. 2017, 40, 429–432. [Google Scholar] [CrossRef]
- De Giorgi, V.; Silvestri, F.; Savarese, I.; Venturi, F.; Scarfì, F.; Trane, L.; Bellerba, F.; Zuccaro, B.; Maio, V.; Massi, D.; et al. Porocarcinoma: An epidemiological, clinical, and dermoscopic 20-year study. Int. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Belin, E.; Ezzedine, K.; Stanislas, S.; Lalanne, N.; Beylot-Barry, M.; Taieb, A.; Vergier, B.; Jouary, T. Factors in the surgical management of primary eccrine porocarcinoma: Prognostic histological factors can guide the surgical procedure. Br. J. Dermatol. 2011, 165, 985–989. [Google Scholar] [CrossRef]
- Brown, C.W., Jr.; Dy, L.C. Eccrine porocarcinoma. Dermatol. Ther. 2008, 21, 433–438. [Google Scholar] [CrossRef]
- Sekine, S.; Kiyono, T.; Ryo, E.; Ogawa, R.; Wakai, S.; Ichikawa, H.; Suzuki, K.; Arai, S.; Tsuta, K.; Ishida, M.; et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J. Clin. Investig. 2019, 129, 3827–3832. [Google Scholar] [CrossRef]
| Head and Neck Skin N (%) | Other Sites N (%) | Statistical Difference p-Value | Overall N (%) | |
|---|---|---|---|---|
| Age at diagnosis | p = 0.358 | |||
| Mean | 66.4 | 67.9 | 67.4 | |
| Std deviation | (σ = 17.6) | (σ = 16.7) | (σ = 17) | |
| Sex | p = 0.005 | |||
| Male | 110 (64) | 200 (51.2) | 310 (55.1) | |
| Female | 62 (36) | 191 (48.8) | 253 (44.9) | |
| Race | p = 0.092 * | |||
| White | 148 (86) | 299 (76.5) | 447 (79.4) | |
| Black | 8 (4.7) | 34 (8.7) | 42 (7.5) | |
| Asian or Pacific Islander | 7 (4.1) | 29 (7.4) | 36 (6.4) | |
| American Indian/Alaska Native | 0 (0) | 1 (0.3) | 1 (0.2) | |
| Unknown ψ | 9 (5.2) | 28 (7.2) | 37 (6.6) | |
| Grade | p = 0.612 * | |||
| I: Well differentiated | 3 (1.7) | 10 (2.6) | 13 (2.3) | |
| II: Moderately differentiated | 9 (5.2) | 25 (6.4) | 34 (6) | |
| III: Poorly differentiated | 13 (7.6) | 20 (5.1) | 33 (5.9) | |
| IV: Undifferentiated; anaplastic | 2 (1.2) | 5 (1.3) | 7 (1.2) | |
| Unknown ψ | 145 (84.3) | 331 (84.7) | 476 (84.5) | |
| Stage | p = 0.433 | |||
| Localized | 120 (69.8) | 265 (67.8) | 385 (68.4) | |
| Regional | 5 (2.9) | 15 (3.8) | 20 (3.6) | |
| Distant | 4 (2.3) | 4 (1) | 8 (1.4) | |
| Unknown ψ | 43 (25) | 107 (27.4) | 150 (26.6) | |
| Tumor size | p = 0.081 | |||
| Mean | 18.8 mm | 24.4 mm | 22.7 mm | |
| Std Deviation | (σ = 18.3) | (σ = 24.7) | (σ = 23.1) | |
| Surgery | p = 0.000 | |||
| No surgery performed | 25 (14.5) | 47 (12) | 72 (12.8) | |
| Excision or destruction of the lesion | 53 (30.8) | 123 (31.5) | 176 (31.3) | |
| Biopsy followed by gross excision | 36 (20.9) | 103 (26.3) | 139 (24.7) | |
| Mohs surgery | 34 (19.8) | 21 (5.4) | 55 (9.8) | |
| Wide excision with margins over 1 cm (microscopically negative) | 24 (14) | 91 (23.3) | 115 (20.4) | |
| Major amputation | 0 (0) | 4 (1) | 4 (0.7) | |
| Unknown ψ | 0 (0) | 2 (0.5) | 2 (0.4) | |
| Radiotherapy | p = 0.841 | |||
| No/unknown | 166 (96.5) | 376 (96.2) | 542 (96.3) | |
| Beam radiation | 6 (3.5) | 15 (3.8) | 21 (3.7) | |
| Chemotherapy | p = 0.731 * | |||
| No/unknown | 170 (98.8) | 385 (98.5) | 555 (98.6) | |
| Yes | 2 (1.2) | 6 (1.5) | 8 (1.4) | |
| Primary site | ||||
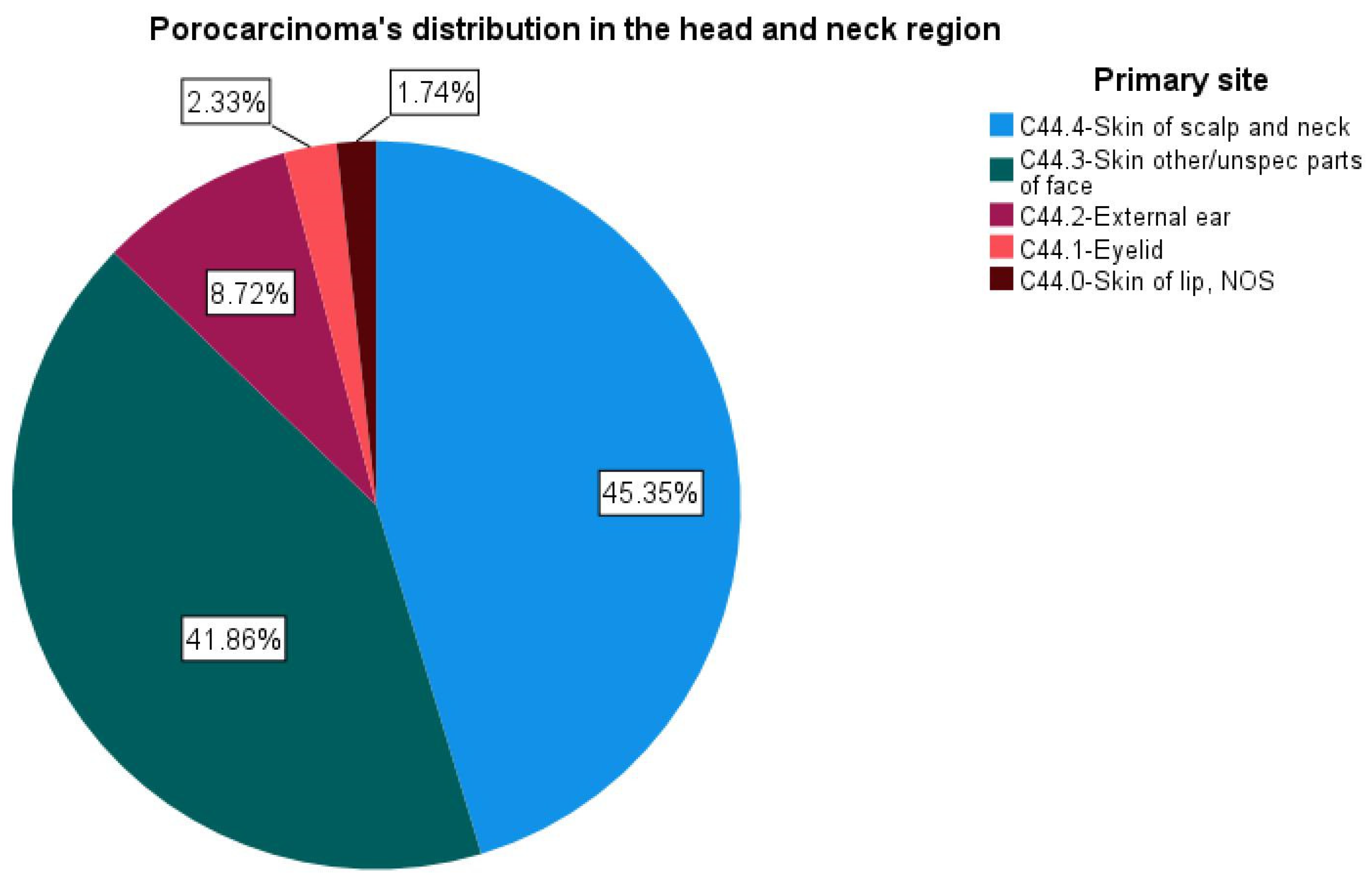
| C44.0—Skin of lip, NOS | 3 (1.7) | 3 (0.5) | ||
| C44.1—Eyelid | 4 (2.3) | 4 (0.7) | ||
| C44.2—External ear | 15 (8.7) | 15 (2.7) | ||
| C44.3—Skin other/unspec parts of face | 72 (41.9) | 72 (12.8) | ||
| C44.4—Skin of scalp and neck | 78 (45.3) | 78 (13.9) | ||
| C44.5—Skin of trunk | 110 (28.1) | 110 (19.5) | ||
| C44.6—Skin of upper limb and shoulder | 79 (20.2) | 79 (14) | ||
| C44.7—Skin of lower limb and hip | 190 (48.6) | 190 (33.7) | ||
| C44.9—Skin, NOS | 8 (2) | 8 (1.4) | ||
| C49.2—Connective, subcutaneous, other soft tissue: lower limb, hip | 1 (0.3) | 1 (0.2) | ||
| C51.9—Vulva, NOS | 1 (0.3) | 1 (0.2) | ||
| C60.9—Penis, NOS | 1 (0.3) | 1 (0.2) | ||
| C63.2—Scrotum, NOS | 1 (0.3) | 1 (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scampa, M.; Merat, R.; Kalbermatten, D.F.; Oranges, C.M. Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival. J. Clin. Med. 2022, 11, 2185. https://doi.org/10.3390/jcm11082185
Scampa M, Merat R, Kalbermatten DF, Oranges CM. Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival. Journal of Clinical Medicine. 2022; 11(8):2185. https://doi.org/10.3390/jcm11082185
Chicago/Turabian StyleScampa, Matteo, Rastine Merat, Daniel F. Kalbermatten, and Carlo M. Oranges. 2022. "Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival" Journal of Clinical Medicine 11, no. 8: 2185. https://doi.org/10.3390/jcm11082185
APA StyleScampa, M., Merat, R., Kalbermatten, D. F., & Oranges, C. M. (2022). Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival. Journal of Clinical Medicine, 11(8), 2185. https://doi.org/10.3390/jcm11082185







