Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anti-Spike Protein Measurement
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 vaccine booster and mortality due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S. Data and Clinical Considerations for Additional Doses in Immunocompromised People; Center for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and cellular immunogenicity of the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in nursing home residents. Clin. Infect. Dis. 2021, 73, 2145–2147. [Google Scholar] [CrossRef]
- Kronbichler, A.; Anders, H.J.; Fernandez-Juárez, G.M.; Floege, J.; Goumenos, D.; Segelmark, M.; Tesar, V.; Turkmen, K.; van Kooten, C.; Bruchfeld, A.; et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol. Dial. Transplant. 2021, 36, 1160–1168. [Google Scholar] [CrossRef]
- Windpessl, M.; Bruchfeld, A.; Anders, H.J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef]
- Betjes, M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef]
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sánchez-Álvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambühl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am. J. Kidney Dis. 2021, 77, 748–756.e1. [Google Scholar] [CrossRef]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Morgan, C.; Kerschmann, R.; Beyer, P.; Dittrich, M.; et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann. Intern. Med. 2021, 175, 371–378. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lee, T.H.; Tian, Y.C.; Lee, C.C.; Fan, P.C.; Chang, C.H. Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e2131749. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Schaier, M.; Nusshag, C.; Töllner, M.; Buylaert, M.; Kälble, F.; Reichel, P.; Grenz, J.; Süsal, C.; Zeier, M.; et al. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines 2021, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021, 100, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Angel-Korman, A.; Peres, E.; Bryk, G.; Lustig, Y.; Indenbaum, V.; Amit, S.; Rappoport, V.; Katzir, Z.; Yagil, Y.; Iaina, N.L.; et al. Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel: The case for a third vaccine dose. Clin. Kidney J. 2022, 15, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dekervel, M.; Henry, N.; Torreggiani, M.; Pouteau, L.M.; Imiela, J.P.; Mellaza, C.; Garnier, A.S.; Dujardin, A.; Asfar, M.; Ducancelle, A.; et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021, 14, 2349–2355. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e1. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Tzukert, K.; Aharon, M.; Pri-Chen, H.; Oster, Y.; Oiknine-Djian, E.; Wolf, D.G.; Dranitzki Elhalel, M. Response to tozinameran (BNT162b2) booster in twice-vaccinated kidney transplant and maintenance dialysis patients. J. Nephrol. 2022, 2–4. [Google Scholar] [CrossRef]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Tillmann, F.P.; Figiel, L.; Ricken, J.; Still, H.; Korte, C.; Plassmann, G.; von Landenberg, P. Evolution of SARS-CoV-2-neutralizing antibodies after two standard dose vaccinations, risk factors for non-response and effect of a third dose booster vaccination in non-responders on hemodialysis: A prospective multi-centre cohort study. J. Clin. Med. 2021, 10, 5113. [Google Scholar] [CrossRef]
- de Meester, J.; de Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; de Vriese, A.S.; NBVN Kidney Registry Group. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: A regionwide registry study. J. Am. Soc. Nephrol. 2021, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Arroyo, R.; Portolés, J.; López-Sánchez, P.; Zalamea, F.; Furaz, K.; Méndez, Á.; Nieto, L.; Sánchez-Hernández, R.; Pizarro, S.; García, A.S.; et al. Rapid decline of anti-SARS-CoV-2 antibodies in patients on haemodialysis: The COVID-FRIAT study. Clin. Kidney J. 2021, 14, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Takazono, T.; Yamamoto, K.; Harada, T.; Funakoshi, S.; Mukae, H.; Nishino, T. Low humoral immune response to the BNT162b2 vaccine against COVID-19 in nursing home residents undergoing hemodialysis: A case–control observational study. Ren. Replace. Ther. 2022, 8, 8. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Forbes, S.; Davari, M.; Gnanasampanthan, S.; Roth, N.; Young, G.; Rajakariar, R.; Cove-Smith, A.; Yaqoob, M.M.; Cutino-Moguel, T.; Mahalingasivam, V.; et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol. Dial. Transplant. 2021, 36, 1292–1297. [Google Scholar] [CrossRef]
- Attias, P.; Sakhi, H.; Rieu, P.; Soorkia, A.; Assayag, D.; Bouhroum, S.; Nizard, P.; El Karoui, K. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021, 99, 1490–1492. [Google Scholar] [CrossRef]
- Cohen, G.; Hörl, W.H. Immune dysfunction in uremia—An update. Toxins 2012, 4, 962–990. [Google Scholar] [CrossRef] [Green Version]
- Beddhu, S.; Bruns, F.J.; Saul, M.; Seddon, P.; Zeidel, M.L. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am. J. Med. 2000, 108, 609–613. [Google Scholar] [CrossRef]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Sood, M.M. Growing understanding of the clinical and serologic effects of COVID-19 vaccines in patients undergoing long-term dialysis. Clin. J. Am. Soc. Nephrol. 2022, 17, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A.; Omicron, S.-C. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.F.; Malik, A.A.; De la Cruz, E.; Jorge, A.; et al. Neutralizing antibodies against the SARS-CoV-2 delta and omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef]
| Patients on HD (n = 279) | Healthcare Workers (n = 189) | p-Value | |
|---|---|---|---|
| Age (years) | 69 ± 11 | 45 ± 13 | <0.001 |
| Sex (male) (%) | 65 | 30 | <0.001 |
| BMI (kg/m2) | 21.3 ± 3.7 | 21.8 ± 4.2 | 0.28 |
| Dialysis vintage (months) | 69 (34–141) | NA | |
| Diabetes mellitus (%) | 44 | 5 | <0.001 |
| Hypertension (%) | 91 | 6 | <0.001 |
| History of ischemic heart diseases (%) | 52 | 0 | <0.001 |
| History of stroke (%) | 22 | 0 | <0.001 |
| Mean KT/V | 1.49 ± 0.30 | NA | |
| White blood cell count (/μL) | 6275 ± 2179 | 6224 ± 1367 | 0.80 |
| Patients on HD (n = 279) | Healthcare Workers (n = 189) | p-Value | |
|---|---|---|---|
| Measurement in October 2021 | |||
| Days since the second dose of BNT162b2 | 91 ± 13 | 138 ± 4 | <0.001 |
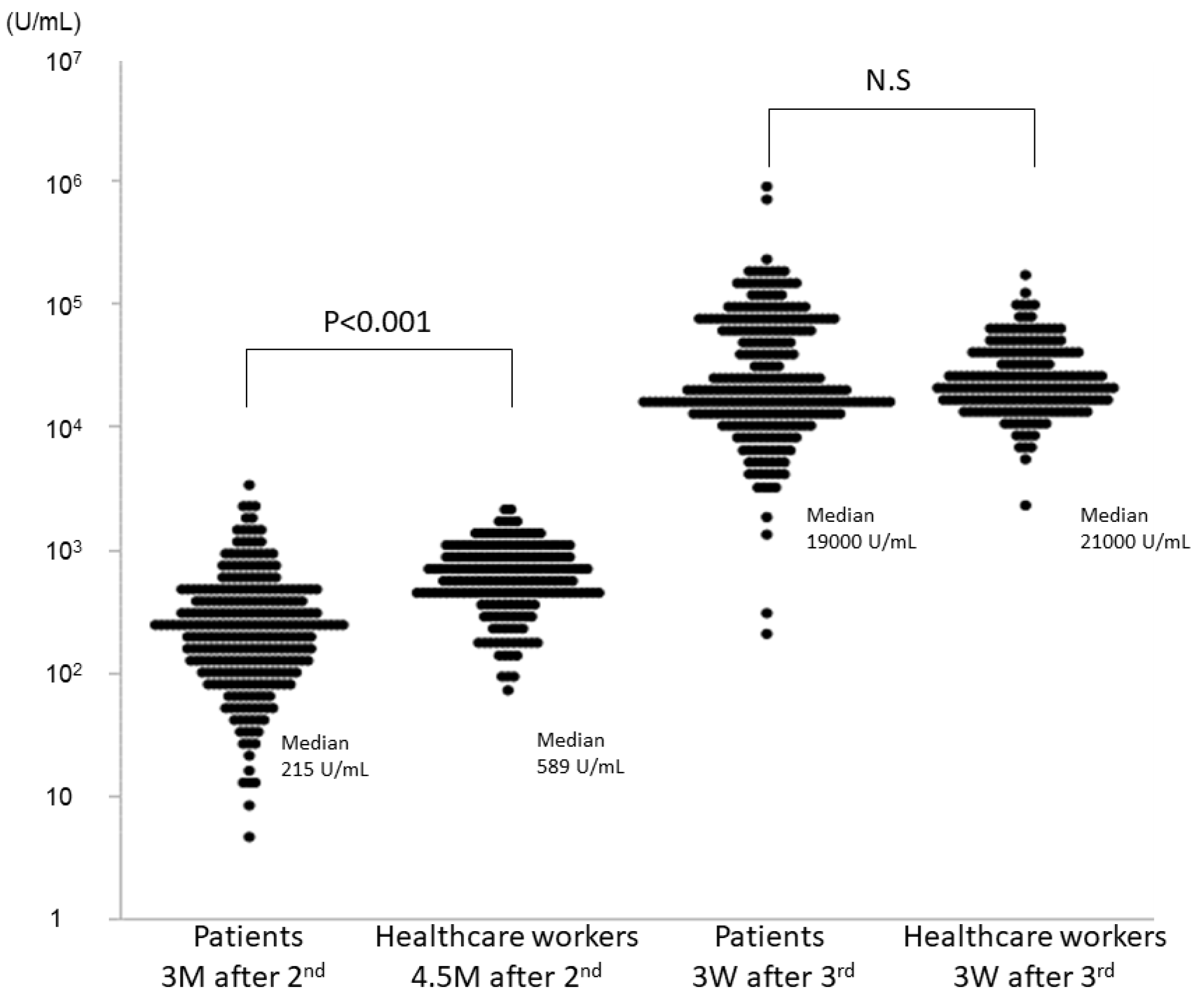
| (1) anti-S IgG antibody levels at the follow-up of the second dose (U/mL) | 215 (103–387) | 589 (396–853) | <0.001 |
| Days between the second dose and the third dose of BNT162b2 (days) | 202 ± 11 | 219 ± 5 | <0.001 |
| Measurement 3 weeks after the third dose | |||
| Days since the third dose | 21 ± 1 | 20 ± 3 | <0.001 |
| (2) anti-S IgG antibody levels at the follow-up of the third dose (U/mL) | 19,000 (12,000–61,000) | 21,000 (15,000–36,500) | 0.90 |
| Increasing rate calculating from (2)/(1) | 129 (59–216) | 37 (25–69) | <0.001 |
| Low-Responder in Patients on HD (n = 51) | Responder in Patients on HD (n = 228) | p-Value | |
|---|---|---|---|
| Age (years) | 69 ± 11 | 69 ± 12 | 0.87 |
| Sex (male) (%) | 63 | 66 | 0.68 |
| BMI (kg/m2) | 22.5 ± 4.1 | 21.7 ± 4.2 | 0.08 |
| Dialysis vintage (months) | 60 (35–156) | 75 (33–141) | 0.50 |
| Diabetes mellitus (%) | 33 | 46 | 0.09 |
| Mean KT/V | 1.43 ± 0.25 | 1.50 ± 0.30 | 0.16 |
| White blood cell count (/μL) | 6815 ± 2272 | 6154 ± 2144 | 0.0502 |
| Hemoglobin (g/dL) | 10.8 ± 1.2 | 10.8 ± 1.0 | 0.96 |
| Albumin (g/dL) | 3.5 ± 0.4 | 3.6 ± 0.4 | 0.14 |
| (1) anti-S IgG antibody levels at the follow-up of the second dose (U/mL) | 46 (33–67) | 246 (155–435) | <0.001 |
| (2) anti-S IgG antibody levels at the follow-up of the third dose (U/mL) | 9900 (4560–17,000) | 21,000 (14,000–66,750) | <0.001 |
| Increasing rate calculating from (2)/(1) | 212 (124–613) | 105 (56–193) | <0.001 |
| Patients on HD | Healthcare Workers | |||
|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | |
| Age | −0.111 | 0.06 | −0.227 | 0.002 |
| Dialysis vintage (months) | 0.209 | <0.001 | NA | |
| Body mass index | 0.115 | 0.06 | 0.208 | 0.01 |
| White blood cell count | 0.094 | 0.12 | 0.080 | 0.35 |
| Hemoglobin | −0.115 | 0.05 | 0.067 | 0.43 |
| Albumin | 0.001 | 0.99 | NA | |
| KT/V | 0.097 | 0.11 | NA | |
| Patients on HD | Healthcare Workers | |||
|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | |
| Age | −0.042 | 0.49 | 0.210 | 0.004 |
| Dialysis vintage (months) | 0.084 | 0.16 | NA | |
| Body mass index | 0.210 | <0.001 | 0.144 | 0.09 |
| White blood cell count | 0.128 | 0.03 | 0.024 | 0.78 |
| Hemoglobin | −0.078 | 0.19 | 0.076 | 0.37 |
| Albumin | −0.112 | 0.06 | NA | |
| KT/V | −0.012 | 0.84 | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, M.; Takazono, T.; Yamaguchi, K.; Tomura, H.; Yamamoto, K.; Harada, T.; Funakoshi, S.; Mukae, H.; Nishino, T. Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis. J. Clin. Med. 2022, 11, 2090. https://doi.org/10.3390/jcm11082090
Kitamura M, Takazono T, Yamaguchi K, Tomura H, Yamamoto K, Harada T, Funakoshi S, Mukae H, Nishino T. Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis. Journal of Clinical Medicine. 2022; 11(8):2090. https://doi.org/10.3390/jcm11082090
Chicago/Turabian StyleKitamura, Mineaki, Takahiro Takazono, Kosei Yamaguchi, Hideshi Tomura, Kazuko Yamamoto, Takashi Harada, Satoshi Funakoshi, Hiroshi Mukae, and Tomoya Nishino. 2022. "Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis" Journal of Clinical Medicine 11, no. 8: 2090. https://doi.org/10.3390/jcm11082090
APA StyleKitamura, M., Takazono, T., Yamaguchi, K., Tomura, H., Yamamoto, K., Harada, T., Funakoshi, S., Mukae, H., & Nishino, T. (2022). Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis. Journal of Clinical Medicine, 11(8), 2090. https://doi.org/10.3390/jcm11082090






