Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Sampling
2.2. Data Collection
2.3. Annexin A1
2.4. Soluble Complement 5a
2.5. Von Willebrand Factor Antigen
2.6. Statistical Analysis
3. Results
3.1. Patient Population
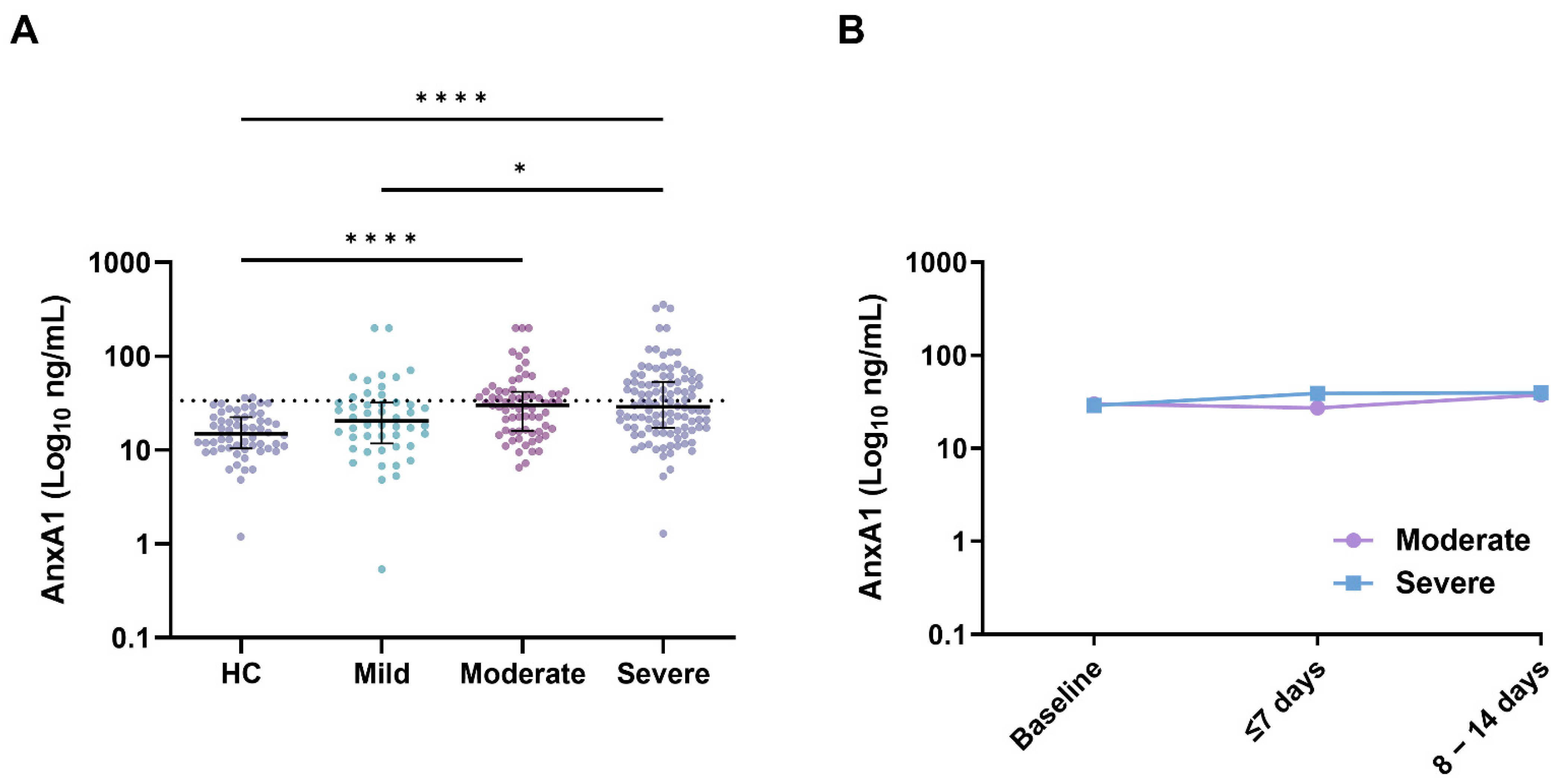
3.2. Annexin A1
3.3. Annexin A1, Inflammation, and Endothelial Damage
3.4. AnxA1 and Clinical Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Busch, M.; Timmermans, S.A.; Nagy, M.; Visser, M.; Huckriede, J.; Aendekerk, J.P.; De Vries, F.; Potjewijd, J.; Jallah, B.; Ysermans, R.; et al. Neutrophils and Contact Activation of Coagulation as Potential Drivers of COVID-19. Circulation 2020, 142, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Chiang, N.; La, M.; Fierro, I.M.; Marullo, S.; Getting, S.J.; Solito, E.; Serhan, C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002, 8, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Nogueira, C.R.C.; Tavares, L.P.; Soriani, F.M.; Lopes, F.; Russo, R.C.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukoc. Biol. 2012, 92, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; D’Acquisto, F.; Pederzoli-Ribeil, M.; Lavagno, L.; Flower, R.J.; Witko-Sarsat, V.; Perretti, M. Annexin 1 Cleavage in Activated Neutrophils. J. Biol. Chem. 2007, 282, 29998–30004. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Milne, I.R.; Bagley, C.J.; Gamble, J.R.; Vadas, M.A.; Pitson, S.M.; Khew-Goodall, Y. A Proinflammatory Role for Proteolytically Cleaved Annexin A1 in Neutrophil Transendothelial Migration. J. Immunol. 2010, 185, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Zouki, C.; Ouellet, S.; Filep, J.G. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J. 2000, 14, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Rhee, H.J.; Ko, J.; Kim, Y.J.; Kim, H.G.; Yang, J.M.; Choi, E.C.; Na, D.S. Inhibition of Cytosolic Phospholipase A2 by Annexin I. J. Biol. Chem. 2001, 276, 15712–15719. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Rosin, N.L.; Arora, R.; Labit, E.; Jaffer, A.; Cao, L.; Farias, R.; Nguyen, A.P.; de Almeida, L.G.N.; Dufour, A.; et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat. Med. 2022, 28, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Canacik, O.; Sabirli, R.; Altintas, E.; Karsli, E.; Karis, D.; Kaymaz, B.; Sabirli, G.T.; Kurt, Ö.; Koseler, A. Annexin A1 as a potential prognostic biomarker for COVID-19 disease: Case–control study. Int. J. Clin. Pract. 2021, 75, e14606. [Google Scholar] [CrossRef] [PubMed]
- Dutch Guidelines for the Treatment of Patients with COVID-19. Available online: https://web.archive.org/web/20200422150127/https://swab.nl/nl/covid-19#to_4 (accessed on 22 April 2020).
- Damazo, A.S.; Sampaio, A.L.; Nakata, C.M.; Flower, R.J.; Perretti, M.; Oliani, S.M. Endogenous annexin A1 counter-regulates bleomycin-induced lung fibrosis. BMC Immunol. 2011, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.P.; Pinho, V.; Teixeira, M.M. Harnessing inflammation resolving-based therapeutic agents to treat pulmonary viral infections: What can the future offer to COVID-19? J. Cereb. Blood Flow Metab. 2020, 177, 3898–3904. [Google Scholar] [CrossRef] [PubMed]
- Gillis, S.; Furie, B.C.; Furie, B. Interactions of neutrophils and coagulation proteins. Semin. Hematol. 1997, 34, 336–342. [Google Scholar] [PubMed]
- Englert, H.; Rangaswamy, C.; Deppermann, C.; Sperhake, J.-P.; Krisp, C.; Schreier, D.; Gordon, E.; Konrath, S.; Haddad, M.; Pula, G.; et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. Ebiomedicine 2021, 67, 103382. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.W.; Lima, F.; Moraes, C.R.P.; Ilich, A.; Huber, S.C.; Barbosa, M.S.; Santos, I.; Palma, A.C.; Nunes, T.A.; Ulaf, R.G.; et al. Contact and intrinsic coagulation pathways are activated and associated with adverse clinical outcomes in COVID-19. Blood Adv. 2022, 6, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Christian, H.; Wheller, S.K.; Aiello, I.; Mugridge, K.G.; Morris, J.F.; Flower, R.J.; Goulding, N.J. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 2000, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]



| Normal Range | Mild (n = 48) | Moderate (n = 68) | Severe (n = 104) | Overall p | |
|---|---|---|---|---|---|
| M/F | 25/23 | 43/25 | 76/28 † | 0.037 | |
| Age, yr. | 62 (±16) | 70 (±12) ‡ | 69 (±12) † | 0.002 | |
| Days from illness onset | 7 (5−11) | 7 (5−14) | 7 (5−14) | 0.963 | |
| SBP, mmHg | 129 (±16) | 137 (±22) | 138 (±25) | 0.068 | |
| DBP, mmHg | 80 (±12) | 79 (±12) | 80 (±14) | 0.885 | |
| Heart rate, bpm | 90 (75−100) | 90 (80−100) | 95 (80−110) *,† | 0.043 | |
| Body temperature, °C | ≤37.9 | 37.7 (±0.9) | 38.1 (±1.0) ‡ | 38.1 (±1.0) † | 0.021 |
| Fever, n (%) | 14 (30) | 39 (58) ‡ | 53 (60) † | 0.002 | |
| Medical history | |||||
| Hypertension, n (%) | 15 (31) | 27 (40) | 34 (33) | 0.951 | |
| Diabetes, n (%) | 11 (23) | 11 (16) | 25 (24) | 0.673 | |
| CVA, n (%) | 7 (15) | 11 (16) | 18 (17) | 0.736 | |
| Cardiac disease, n (%) | 13 (27) | 24 (35) | 31 (30) | 0.899 | |
| COPD/asthma, n (%) | 6 (13) | 16 (24) | 11 (11) | 0.418 | |
| None, n (%) | 12 (25) | 15 (22) | 27 (26) | 0.804 | |
| Laboratory parameters | |||||
| Platelets, ×109/L | 130−350 | 195 (164−292) | 202 (143−260) | 211 (168−246) | 0.795 |
| Leukocytes, ×109/L | 3.5−11.0 | 6.0 (5.4−8.4) | 6.3 (4.7−8.7) | 7.4 (5.8−9.9) † | 0.009 |
| Neutrophils, ×109/L | 1.4−7.7 | 4.6 (3.6−6.2) | 5.0 (3.4−7.3) | 5.9 (4.7−8.1) † | <0.001 |
| Lymphocytes, ×109/L | 1.1−4.0 | 1.1 (0.7−1.6) | 0.8 (0.6−1.2) ‡ | 0.7 (0.5−1.1) † | 0.002 |
| NL-ratio | 4.6 (2.8−6.6) | 6.0 (3.9–9.0) ‡ | 8.7 (5.3–12.2) *,† | <0.001 | |
| AST, U/L | <35 | 38 (27−56) | 49 (37−64) ‡ | 55 (40−80) † | <0.001 |
| LDH, U/L | <250 | 256 (205−339) | 328 (266−451) ‡ | 451 (358−595) *,† | <0.001 |
| Serum creatinine, µmol/L | 60−115 | 83 (62−106) | 88 (75−119) | 91 (73−120) | 0.254 |
| Albumin, g/L | 32.0−47.0 | 34 (31−38) | 33 (30−36) | 29 (26−32) *,† | <0.001 |
| CRP, mg/L | <10 | 56 (16−95) | 66 (39−123) | 98 (54−174) *,† | <0.001 |
| C5a, ng/mL | ≤21.1 | 15.3 (9.0−25.4) | 21.8 (16.2−30.7) ‡ | 21.8 (10.8−30.7) † | 0.025 |
| High C5a, n/N | 25/41 | 50/56 ‡ | 70/95 * | 0.005 | |
| AnxA1, ng/mL | ≤33.8 | 20.4 (11.8−32.2) | 30.1 (16.0−42.0) | 28.9 (17.3−53.6) † | 0.025 |
| High AnxA1, n (%) | 11 (23) | 28 (41) ‡ | 46 (44) † | 0.023 |
| Univariable | OR (95% CI) | p-Value | AUC (95% CI) |
|---|---|---|---|
| Thrombotic events | 1.064 (1.003–1.129) | 0.040 | 0.638 (0.535–0.741) |
| ICU admission | 1.052 (0.997–1.111) | 0.065 | 0.611 (0.531–0.691) |
| 28-day mortality | 1.050 (0.982–1.122) | 0.115 | 0.627 (0.514–0.740) |
| Multivariable * | OR (95% CI) | p-value | AUC (95% CI) |
| Thrombotic events * | 1.067 (1.002–1.135) | 0.042 | 0.729 (0.629–0.829) |
| ICU admission # | 1.043 (0.967–1.125) | 0.280 | 0.759 (0.690–0.828) |
| 28-day mortality ± | 0.993 (0.923–1.068) | 0.851 | 0.765 (0.690–0.839) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, M.H.; Timmermans, S.A.M.E.G.; Aendekerk, J.P.; Ysermans, R.; Amiral, J.; Damoiseaux, J.G.M.C.; Reutelingsperger, C.P.; Paassen, P.v. Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19. J. Clin. Med. 2022, 11, 7486. https://doi.org/10.3390/jcm11247486
Busch MH, Timmermans SAMEG, Aendekerk JP, Ysermans R, Amiral J, Damoiseaux JGMC, Reutelingsperger CP, Paassen Pv. Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19. Journal of Clinical Medicine. 2022; 11(24):7486. https://doi.org/10.3390/jcm11247486
Chicago/Turabian StyleBusch, Matthias H., Sjoerd A. M. E. G. Timmermans, Joop P. Aendekerk, Renée Ysermans, Jean Amiral, Jan G. M. C. Damoiseaux, Chris P. Reutelingsperger, and Pieter van Paassen. 2022. "Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19" Journal of Clinical Medicine 11, no. 24: 7486. https://doi.org/10.3390/jcm11247486
APA StyleBusch, M. H., Timmermans, S. A. M. E. G., Aendekerk, J. P., Ysermans, R., Amiral, J., Damoiseaux, J. G. M. C., Reutelingsperger, C. P., & Paassen, P. v. (2022). Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19. Journal of Clinical Medicine, 11(24), 7486. https://doi.org/10.3390/jcm11247486






