Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Determination of Biomarkers of Pro-Inflammatory Cytokines and Endothelial Damage
2.3. Statistical Analysis
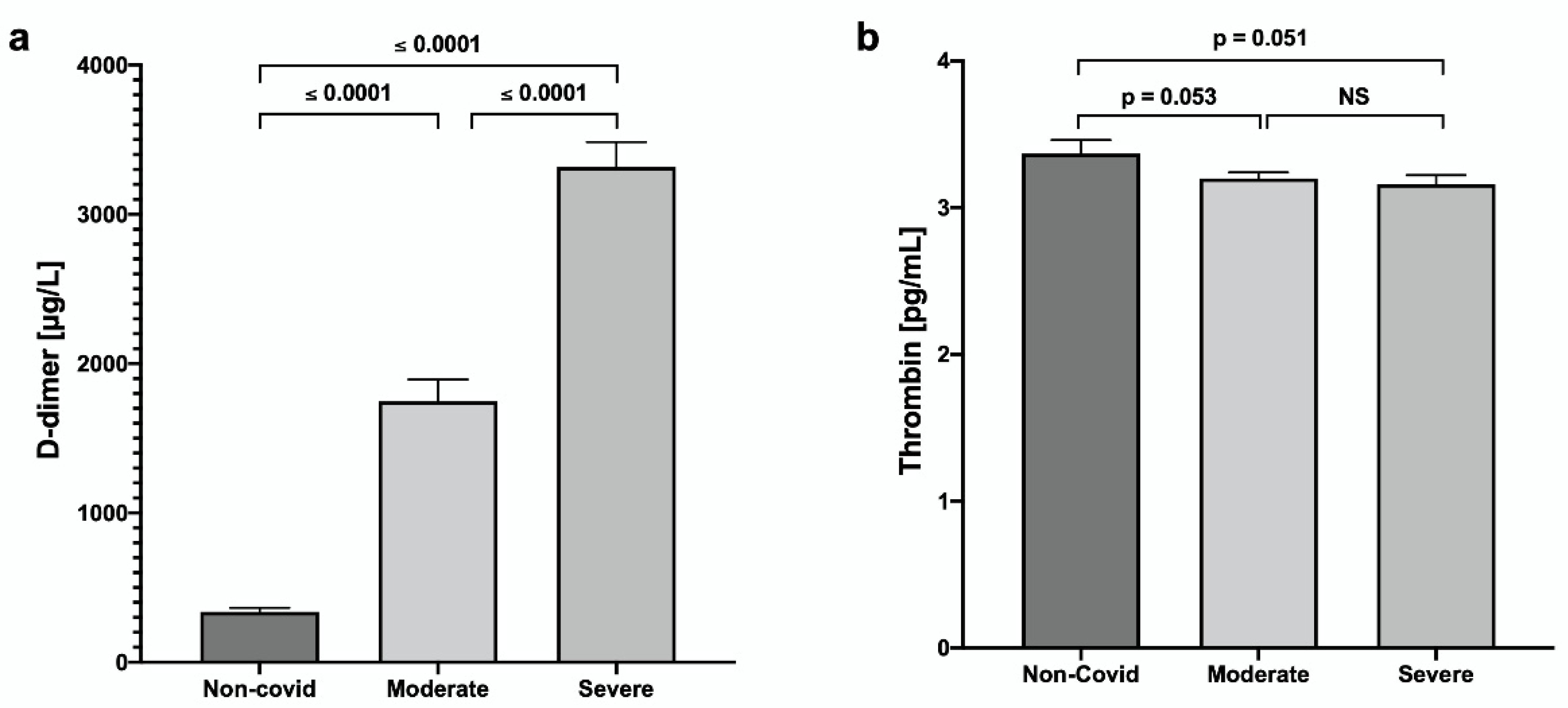
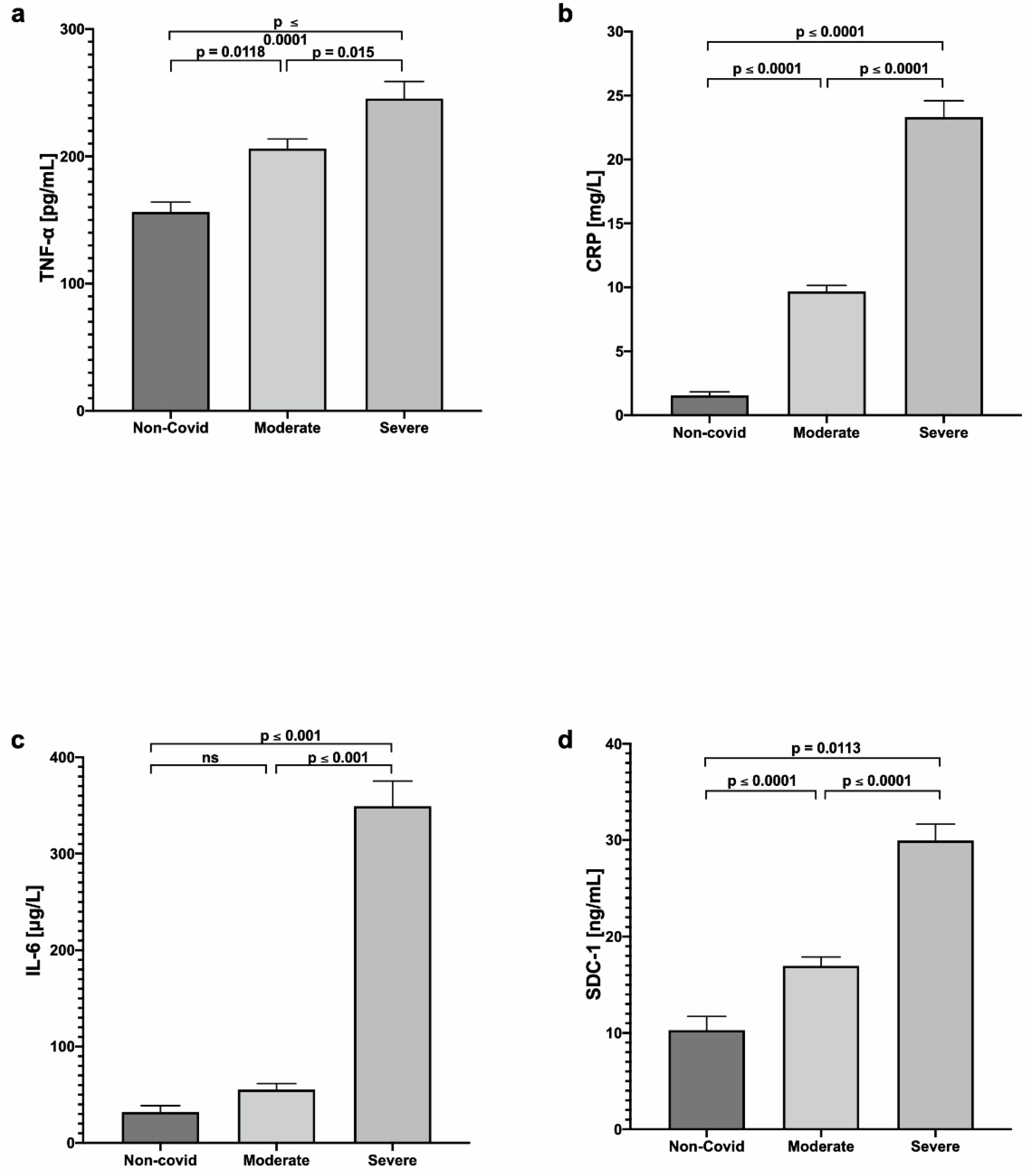
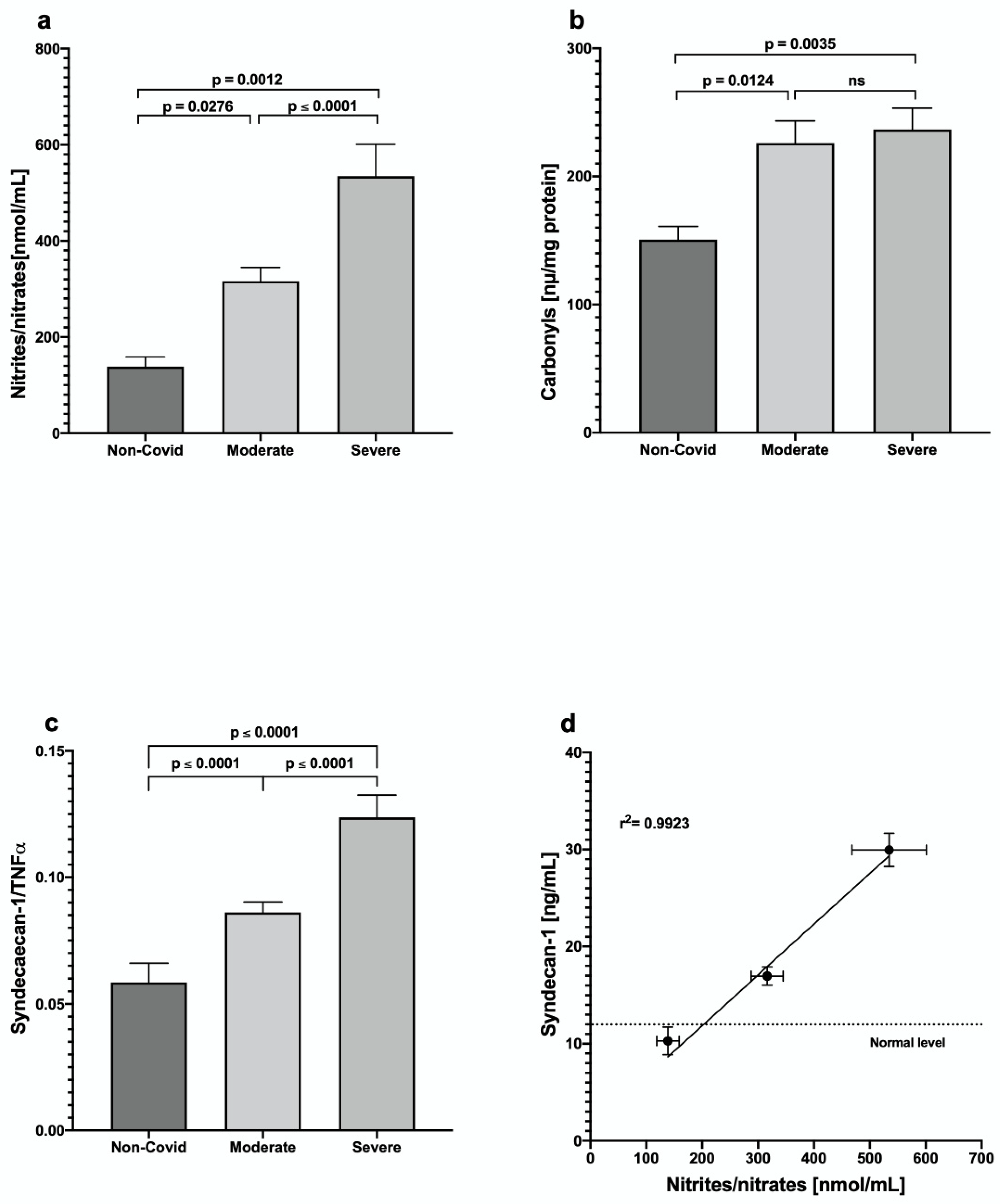
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Bhavana, V.; Thakor, P.; Singh, S.B.; Mehra, N.K.J.L.S. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020, 261, 118336. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Mcgoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Qu, J.; Cheng, Y.; Wu, W.; Yuan, L.; Liu, X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front. Cell Dev. Biol. 2021, 9, 730621. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Chen, Y.; Ma, J.; Yang, Y.; Aodeng, S.; Cui, Q.; Wen, K.; Xiao, M.; Xie, J.; et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021, 27, 151. [Google Scholar] [CrossRef]
- Suzuki, K.; Okada, H.; Sumi, K.; Tomita, H.; Kobayashi, R.; Ishihara, T.; Suzuki, A. Serum syndecan-1 reflects organ dysfunction in critically ill patients. Sci. Rep. 2021, 11, 8864. [Google Scholar] [CrossRef]
- Maruhashi, T.; Higashi, Y. Pathophysiological association of endothelial dysfunction with fatal outcome in COVID-19. Int. J. Mol. Sci. 2021, 22, 5131. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Nguyen, M.H. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e51. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. International journal of infectious diseases. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 95, 304–307. [Google Scholar]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Shumilov, D.S.; Lysenkov, S.P.; Muzhenya, D.V.; Tuguz, A.R.; Urakova, T.U.; Thakushinov, I.A. Participation of nitrogen oxide and its metabolites in the genesis of hyperimmune inflammation in COVID-19. Chin. J. Physiol. 2021, 64, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 711003. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Wang, J.; Zhang, Q. Roles of Endovascular Calyx Related Enzymes in Endothelial Dysfunction and Diabetic. Vasc. Complicat. 2020, 11, 590614. [Google Scholar]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; Dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef]
- Karampoor, S.; Zahednasab, H.; Farahmand, M.; Mirzaei, R.; Zamani, F.; Tabibzadeh, A.; Keyvani, H. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef]
- Hatanaka, K.; Ito, T.; Madokoro, Y.; Kamikokuryo, C.; Niiyama, S.; Yamada, S.; Kakihana, Y. Circulating Syndecan-1 as a Predictor of Persistent Thrombocytopenia and Lethal Outcome: A Population Study of Patients with Suspected Sepsis Requiring Intensive Care. Front. Cardiovasc. Med. 2021, 8, 730553. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Prati, B.; Guida, L.; Parise, A.; Cerundolo, N.; Meschi, T. The Clinical Significance of Procalcitonin Elevation in Patients over 75 Years Old Admitted for COVID-19 Pneumonia. Mediat. Inflamm. 2021, 2021, 5593806. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Mangoni, A.A.; Dettori, P.; Nasrallah, G.K.; Pintus, G.; Zinellu, A. D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front. Public Health 2020, 8, 432. [Google Scholar] [CrossRef]
- Poudel, A.; Poudel, Y.; Adhikari, A.; Aryal, B.B.; Dangol, D.; Bajracharya, T.; Maharjan, A.; Gautam, R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE 2021, 16, e0256744. [Google Scholar] [CrossRef] [PubMed]
- Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Franciscis, S.; Serra, R. Cardiovascular disease as a biomarker for an increased risk of COVID-19 infection and related poor prognosis. Biomark. Med. 2020, 14, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Visco, V.; Gambardella, J.; Ferruzzi, G.J.; Rispoli, A.; Rusciano, M.R.; Ciccarelli, M. Cardiovascular implications of miRNAs in COVID-19. J. Pharmacol. Exp. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
| Non-COVID-19 n = 15 | Moderate n = 37 | Severe n = 29 | |
|---|---|---|---|
| Age (years) | 53.13 ± 90 | 60.49 ± 14.62 | 65.42 ± 12.07 |
| Symptoms, n (%) | |||
| Fever | 0 | 19 (51.35) | 16 (55.17) |
| Cephalea | 1 | 14 (37.83) | 7 (24.13) |
| Congestion | 0 | 2 (5.40) | 1 (3.44 |
| Rhinorrhea | 0 | 8 (21.62) | 3 (10.34) |
| Cough | 0 | 23 (62.16) | 21 (72.41) |
| Odynophagia | 0 | 6 (16.21) | 6 (20.68) |
| Arthralgias | 0 | 25 (67.56) | 13 (44.82) |
| Myalgia | 0 | 23 (62.16) | 13 (44.82) |
| Dyspnea | 0 | 30 (8.10) | 26 (89.65) |
| Diarrhea | 0 | 5 (13.51) | 5 (17.24) |
| Characteristics | Moderate n = 37 | Severe n = 29 |
|---|---|---|
| Days of treatments * | 21.76 ± 13.25 | 23.42 ± 11.26 |
| Prone positioning, n (%) | 11 (29.72) | 23 (79.31) |
| LOMV, median [IQR] ** | 0.00 | 15.23 ± 11.15 |
| ICU LOS, median [IQR] ** | 0.30 ± 1.81 | 5.81 ± 9.2 |
| Hospital LOS median [IQR] ** | 21.73 ± 13.25 | 22.81± 11.20 |
| Comorbidities, n (%) | ||
| Diabetes mellitus | 16 (43.24) | 8 (27.58) |
| Hypertension | 13 (35.13) | 8 (27.58) |
| Obesity | 4 (10.81) | 3 (10.34) |
| Chronic kidney disease | 1 (2.70) | 0 |
| COVID-19 specific treatments n (%) | ||
| Azithromycin | 12 (32.43) | 9 (31.03) |
| Ceftriaxone | 13 (35.13) | 15 (51.72) |
| Levofloxacin | 13 (35.13) | 12 (41.37) |
| Clarithromycin | 1 (2.70) | 0 |
| Budesonide | 5 (13.51) | 6 (20.68) |
| Dexamethasone | 26 (70.27) | 24 (82.75) |
| Enoxaparin | 30 (81.08) | 22 (75.86) |
| Ivermectin | 11 (29.72) | 7 (24.13) |
| Methylprednisolone | 5 (13.51) | 15 (51.72) |
| Hydrocortisone | 2 (5.40) | 2 (6.89) |
| Prednisone | 2 (5.40) | 0 |
| Parameter | Non-COVID-19 | Moderate | Severe |
|---|---|---|---|
| Glucose (mg/dL) | 149.75 ± 43.00 | 160.24 ± 70.94 | 179.95 ± 49.51 |
| Urea (mg/dL) | 65.80 ± 6.42 | 42.07 ± 16.28 | 65.56 ± 20.08 |
| Creatinine (mg/dL) | 2.37 ± 0.47 | 0.85 ± 0.28 | 1.48 ± 0.54 |
| Uric acid (mg/dL) | 6.60 ± 1.78 | 5.33 ± 1.66 | 7.01 ± 1.13 |
| Cholesterol (mg/dL) | 155.13 ± 34.71 | 152.3 ± 45.70 | 142.58 ± 33.51 |
| Triglycerides (mg/dL) | 139.25 ± 34.20 | 166.21 ± 60.14 | 161.17 ± 52.34 |
| HDL-c (mg/dL) | 33.73 ± 4.22 | 36.44 ± 6.34 | 38.21 ± 6.29 |
| LDL-c (mg/dL) | 96.05 ± 25.15 | 103.33 ± 33.41 | 96.19 ± 32.79 |
| BUN (mg/dL) | 22.44 ± 3.85 | 21.55 ± 8.60 | 30.39 ± 12.34 |
| Parameter | Non-COVID-19 | Moderate | p # | Severe | p |
|---|---|---|---|---|---|
| DBIL, mg/dL | 0.28 ± 0.07 | 0.34 ± 0.12 | NS | 0.38 ± 0.18 | NS |
| IBIL, mg/dL | 0.40 ± 0.05 | 0.32 ± 0.11 | NS | 0.27 ± 0.12 | 0.0100 # |
| Total bilirubin, mg/dL | 0.69 ± 0.07 | 0.66 ± 0.26 | NS | 0.65 ± 0.28 | NS |
| ALT, U/L | 27.65 ± 5.54 | 69.03 ± 22.06 | 0.0001 | 42.27 ± 23.95 | 0.0001 ## |
| AST, U/L | 40.53 ± 2.82 | 66.20 ± 10.04 | 0.0008 | 44.64 ± 24.85 | 0.0001 ## |
| Alkaline phosphatase, U/L | 154.41 ± 25.46 | 109.04 ± 37.68 | 0.0060 | 131.14 ± 37.87 | 0.0100 # |
| GGT, U/L | 146.05 ± 34.83 | 130.24 ± 41.82 | NS | 175.27 ± 37.09 | 0.0001 ## |
| LDH, U/L | 294.00 ± 31.11 | 377.55 ± 81.68 | 0.0001 | 601.10 ± 182.62 | 0.0001 ## |
| Total Protein, mg/dL | 5.70 ± 0.95 | 6.37 ± 0.85 | NS | 5.91 ± 0.69 | NS |
| Albumin, g/dL | 3.18 ± 0.71 | 3.59 ± 0.53 | NS | 3.19 ± 0.58 | 0.0100 ## |
| Globulin, g/dL | 2.60 ± 0.59 | 2.78 ± 0.59 | NS | 2.70 ± 0.33 | NS |
| PCT, ng/mL | 0.36 ± 0.16 | 0.27 ± 0.10 | NS | 2.75 ± 0.44 | 0.0001 # 0.0001 ## |
| Platelets, per mL | 244.00 ± 46.29 | 250.20 ± 94.87 | NS | 214.71 ± 43.46 | NS # NS ## |
| Ferritin, mg/L | 365.88 ± 56.05 | 924.69 ± 270.81 | 0.0001 | 858.07 ± 257.37 | 0.0001 # NS ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía, L.; Nájera, N.; Martínez, F.d.J.; Díaz-Chiguer, D.; Jiménez-Ponce, F.; Ortiz-Flores, M.; Villarreal, F.; Ceballos, G. Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients. J. Clin. Med. 2022, 11, 7436. https://doi.org/10.3390/jcm11247436
Munguía L, Nájera N, Martínez FdJ, Díaz-Chiguer D, Jiménez-Ponce F, Ortiz-Flores M, Villarreal F, Ceballos G. Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(24):7436. https://doi.org/10.3390/jcm11247436
Chicago/Turabian StyleMunguía, Levy, Nayelli Nájera, Felipe de Jesús Martínez, Dylan Díaz-Chiguer, Fiacro Jiménez-Ponce, Miguel Ortiz-Flores, Francisco Villarreal, and Guillermo Ceballos. 2022. "Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients" Journal of Clinical Medicine 11, no. 24: 7436. https://doi.org/10.3390/jcm11247436
APA StyleMunguía, L., Nájera, N., Martínez, F. d. J., Díaz-Chiguer, D., Jiménez-Ponce, F., Ortiz-Flores, M., Villarreal, F., & Ceballos, G. (2022). Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients. Journal of Clinical Medicine, 11(24), 7436. https://doi.org/10.3390/jcm11247436






