Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Variables
- ELp: the absence of IIA EL at the end of procedure;
- EL1: the absence of IIA EL at 1 month;
- EL2: the absence of IIA EL at 6 months;
- EL3: the absence of IIA EL at 1 year;
- EL4: the absence of IIA EL at more than 1 year;
- Mortality: any death related to IIA EL;
- Re-intervention: any re-intervention related to IIA EL.
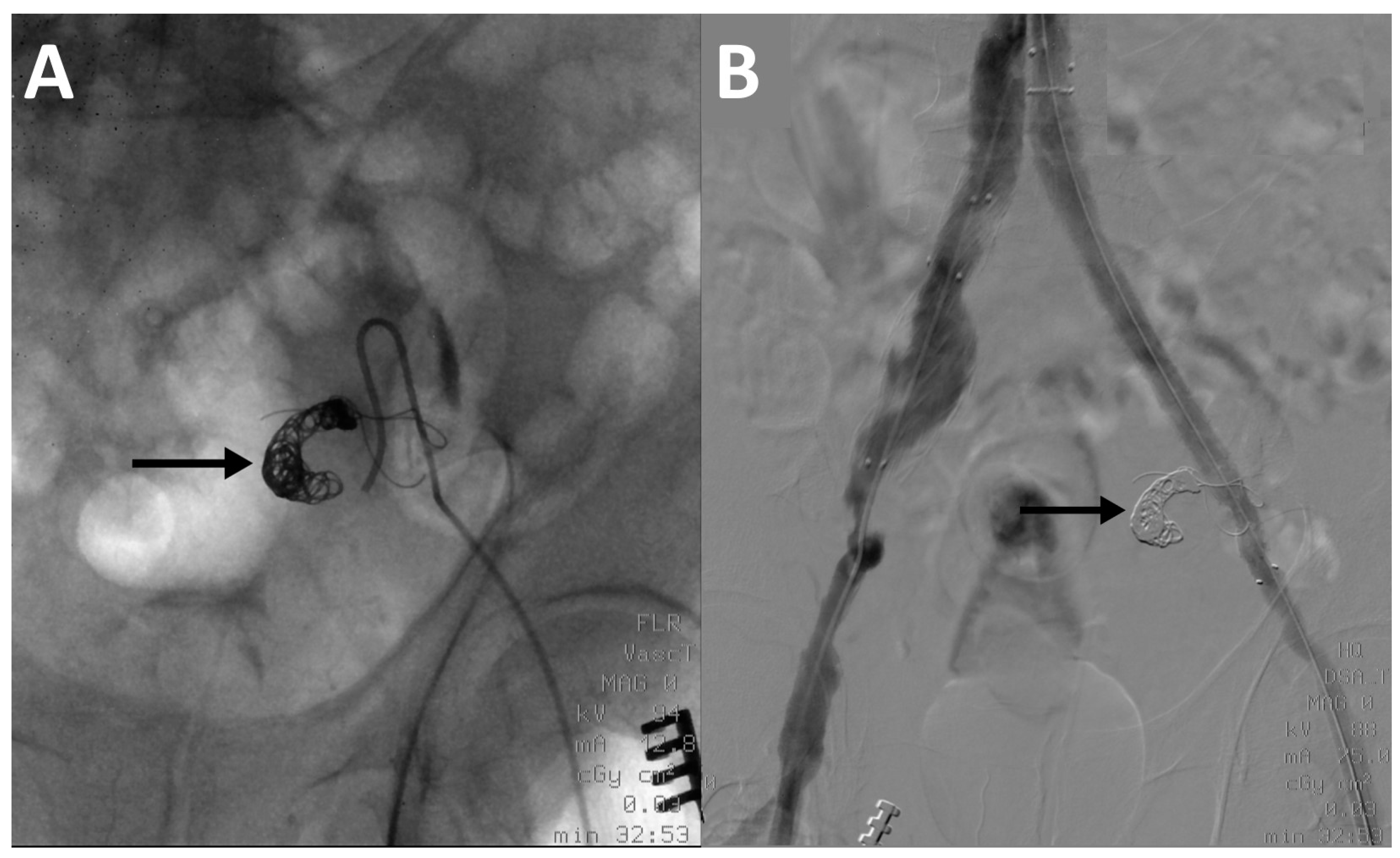
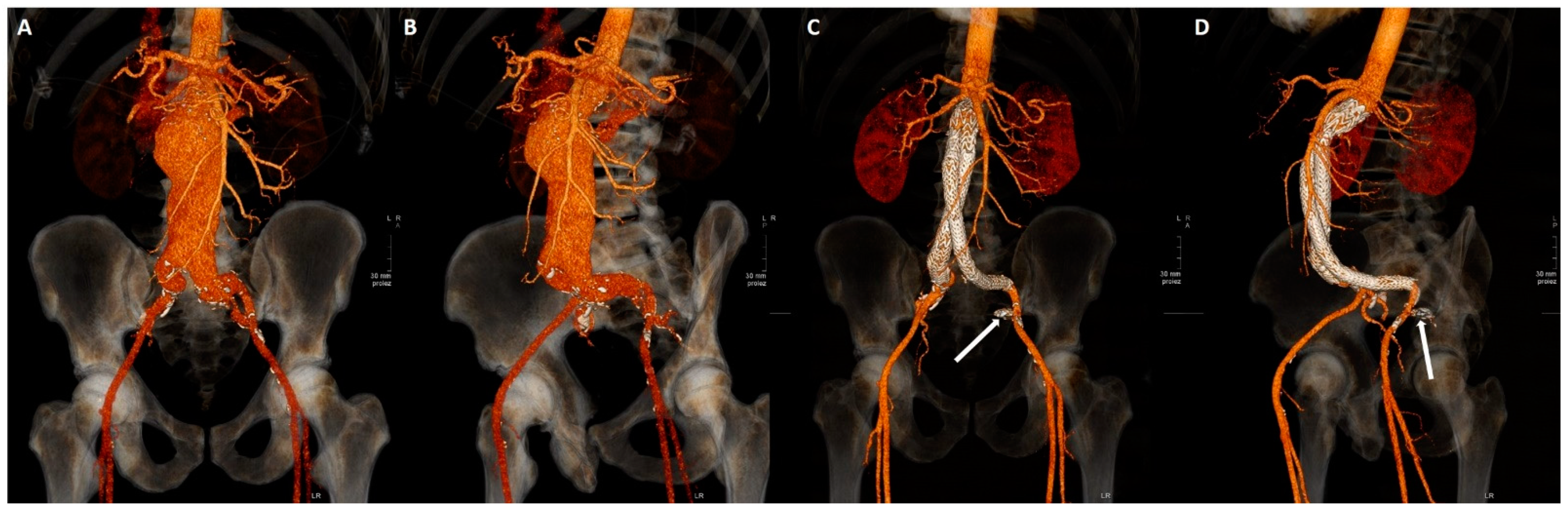
2.3. Embolization Technique
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral Intraluminal Graft Implantation for Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, D.C.; Wilcox, C.; Whitehurst, L.; Cox, A.; Williams, I.M.; Twine, C.P. British Society of Endovascular Therapy (BSET) Systematic Review and Meta-Analysis of the Effect of Internal Iliac Artery Exclusion for Patients Undergoing EVAR. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.S.; Pipinos, I.I. Isolated Iliac Artery Aneurysms. Semin. Vasc. Surg. 2005, 18, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Katsargyris, A.; Oikonomou, K.; Klonaris, C.; Bal, A.; Yanar, F.; Verhoeven, E.L. Common Iliac and Hypogastric Aneurysms: Open and Endovascular Repair. J. Cardiovasc. Surg. 2015, 56, 249–255. [Google Scholar]
- Karthikesalingam, A.; Hinchliffe, R.J.; Holt, P.J.E.; Boyle, J.R.; Loftus, I.M.; Thompson, M.M. Endovascular Aneurysm Repair with Preservation of the Internal Iliac Artery Using the Iliac Branch Graft Device. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 285–294. [Google Scholar] [CrossRef]
- Kurose, S.; Matsubara, Y.; Yoshino, S.; Nakayama, K.; Yamashita, S.; Morisaki, K.; Furuyama, T.; Mori, M. Influence of Internal Iliac Artery Embolization during Endovascular Aortic Repair Regarding Postoperative Sarcopenia and Midterm Survival. Ann. Vasc. Surg. 2021, 74, 148–157. [Google Scholar] [CrossRef]
- Kim, W.C.; Jeon, Y.S.; Hong, K.C.; Kim, J.Y.; Cho, S.G.; Park, J.Y. Internal Iliac Artery Embolization during an Endovascular Aneurysm Repair with Detachable Interlock Microcoils. Korean J. Radiol. 2014, 15, 613. [Google Scholar] [CrossRef]
- Kjellin, P.; Pärsson, H.; Lindgren, H.I.V. Onyx Embolization for Occlusion of the Proximal Internal Iliac Artery During EVAR in Patients with Unsuitable Landing Zones in the Common Iliac Artery. Cardiovasc. Interv. Radiol. 2019, 42, 956–961. [Google Scholar] [CrossRef]
- Kouvelos, G.N.; Koutsoumpelis, A.; Peroulis, M.; Matsagkas, M. In Endovascular Aneurysm Repair Cases, When Should You Consider Internal Iliac Artery Embolization When Extending a Stent into the External Iliac Artery? Interact. CardioVasc. Thorac. Surg. 2014, 18, 821–824. [Google Scholar] [CrossRef][Green Version]
- Kouvelos, G.N.; Giannoukas, A.D.; Matsagkas, M. Commentary: Internal Iliac Artery Embolization During EVAR: Time to Move Forward. J. Endovasc. 2017, 24, 57–58. [Google Scholar] [CrossRef]
- Kang, J.; Chung, B.-H.; Hyun, D.-H.; Park, Y.-J.; Kim, D.-I. Clinical Outcomes after Internal Iliac Artery Embolization Prior to Endovascular Aortic Aneurysm Repair. Int. Angiol. 2020, 39, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Baba, H.; Okamoto, K.; Joo, K.; Ochiai, Y.; Tokunaga, S. Unilateral Embolization of the Internal Iliac Artery for Endovascular Aortic Repair Does Not Induce Gluteal Muscle Atrophy. Ann. Vasc. Surg. 2021, 73, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Kitamura, T.; Mishima, T.; Nakajima, R.; Tamura, Y.; Horikoshi, R.; Araki, H.; Yakuwa, K.; Tomoyasu, T.; Okamura, T.; et al. Gluteal Blood Flow Monitoring in Endovascular Aneurysm Repair With Internal Iliac Artery Embolization. Circ. J. 2021, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Johnson, P.; Chen, Z.; Newsome, J.; Bercu, Z.; Findeiss, L.K.; Dariushnia, S.; Rajani, R.; Kokabi, N. A Meta-Analysis of Comparative Outcome and Cost-Effectiveness of Internal Iliac Artery Embolization with Vascular Plug Versus Coil. Cardiovasc. Interv. Radiol. 2020, 43, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Lanza, C.; Marra, P.; Colarieti, A.; Panzeri, M.; Augello, L.; Gusmini, S.; Salvioni, M.; De Cobelli, F.; Del Maschio, A. Transcatheter Embolization with Squid, Combined with Other Embolic Agents or Alone, in Different Abdominal Diseases: A Single-Center Experience in 30 Patients. CVIR Endovasc. 2019, 2, 8. [Google Scholar] [CrossRef]
- Lucatelli, P.; Corona, M.; Teodoli, L.; Nardis, P.; Cannavale, A.; Rocco, B.; Trobiani, C.; Cipollari, S.; Zilahi de Gyurgyokai, S.; Bezzi, M.; et al. Use of Phil Embolic Agent for Bleeding in Non-Neurological Interventions. J. Clin. Med. 2021, 10, 701. [Google Scholar] [CrossRef]
- Chun, J.-Y.; Mailli, L.; Abbasi, M.A.; Belli, A.-M.; Gonsalves, M.; Munneke, G.; Ratnam, L.; Loftus, I.M.; Morgan, R. Embolization of the Internal Iliac Artery Before EVAR: Is It Effective? Is It Safe? Which Technique Should Be Used? Cardiovasc. Interv. Radiol. 2014, 37, 329–336. [Google Scholar] [CrossRef]
- Kotoku, A.; Ogawa, Y.; Chiba, K.; Maruhashi, T.; Mimura, H.; Miyairi, T.; Nishimaki, H. Clinical Utility of Coil in Plug Method (CIP) for Internal Iliac Artery Embolization during Endovascular Aortic Aneurysm Repair. Ann. Vasc. Dis. 2020, 13, 269–272. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Nassef, A.H.; Antoniou, S.A.; Loh, C.Y.Y.; Turner, D.R.; Beard, J.D. Endovascular Treatment of Isolated Internal Iliac Artery Aneurysms. Vascular 2011, 19, 291–300. [Google Scholar] [CrossRef]
- Mulay, S.; Geraedts, A.C.M.; Koelemay, M.J.W.; Balm, R.; Mulay, S.; Balm, R.; Elshof, J.W.; Elsman, B.H.P.; Hamming, J.F.; Koelemay, M.J.W.; et al. Type 2 Endoleak With or Without Intervention and Survival After Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 779–786. [Google Scholar] [CrossRef]
- Van Marrewijk, C.; Buth, J.; Harris, P.L.; Norgren, L.; Nevelsteen, A.; Wyatt, M.G. Significance of Endoleaks after Endovascular Repair of Abdominal Aortic Aneurysms: The EUROSTAR Experience. J. Vasc. Surg. 2002, 35, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Timaran, C.H.; Ohki, T.; Rhee, S.J.; Veith, F.J.; Gargiulo, N.J.; Toriumi, H.; Malas, M.B.; Suggs, W.D.; Wain, R.A.; Lipsitz, E.C. Predicting Aneurysm Enlargement in Patients with Persistent Type II Endoleaks. J. Vasc. Surg. 2004, 39, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Shao, J.; Xu, F.; Chen, Y.; Liu, B.; Zheng, Y. Single-Center Experience in the Endovascular Management of the Combination of Isolated Common and Internal Iliac Artery Aneurysms. Front. Surg. 2021, 8, 693233. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Tavlas, E.; Papadopoulos, G.; Galanakis, N.; Tsetis, D.; Ioannou, C.V. Embolization or Simple Coverage to Exclude the Internal Iliac Artery During Endovascular Repair of Aortoiliac Aneurysms? Systematic Review and Meta-Analysis of Comparative Studies. J. Endovasc. 2017, 24, 47–56. [Google Scholar] [CrossRef]
- Papazoglou, K.O.; Sfyroeras, G.S.; Zambas, N.; Konstantinidis, K.; Kakkos, S.K.; Mitka, M. Outcomes of Endovascular Aneurysm Repair with Selective Internal Iliac Artery Coverage without Coil Embolization. J. Vasc. Surg. 2012, 56, 298–303. [Google Scholar] [CrossRef]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus Open Repair of Abdominal Aortic Aneurysm in 15-Years’ Follow-up of the UK Endovascular Aneurysm Repair Trial 1 (EVAR Trial 1): A Randomised Controlled Trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef]
- Chen, R.J.; Vaes, R.H.D.; Qi, S.D.; Westcott, M.; Robinson, D.R. Modalities of Endovascular Management for Internal Iliac Artery Aneurysms. ANZ J. Surg. 2021, 91, 2397–2403. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]

| Variable | N | Value | Range | Variable | N | Value | Range |
|---|---|---|---|---|---|---|---|
| Age | 49 | secondary EL (1 month) | 36 | ||||
| median; IQR | 76 (69.5–81.5) | 46–88 | no | 26 (72.22%) | - | ||
| Sex | 49 | type 1 | 2 (5.56%) | ||||
| M | 48 (97.96%) | - | type 2 | 8 (22.22%) | |||
| F | 1 (2.04%) | T2 (6 months) | 34 | ||||
| Laterality | 49 | no | 22 (70.97%) | - | |||
| monolateral | 46 (93.88%) | - | type 1 | 1 (3.23%) | |||
| bilateral | 3 (6.12%) | type 2 | 8 (25.81%) | ||||
| Branch | 49 | T3 (1 year) | 27 | ||||
| Embolization only | 43 (87.76%) | - | no | 22 (81.48%) | - | ||
| Embolization + branch | 6 (12.24%) | type 1 | 0 (0%) | ||||
| Device | 48 | type 2 | 5 (18.52%) | ||||
| coils | 27 (56.25%) | T4 (>1 year) | 12 | ||||
| plug | 19 (39.58%) | - | no | 9 (75%) | - | ||
| Coils + plug | 2 (4.17%) | type 1 | 1 (8.3%) | ||||
| Execution Timing | 49 | type 2 | 2 (16.7%) | ||||
| contestual | 46 (93.9%) | - | How many years after | 12 | |||
| later stage | 3 (6.1%) | mean; SD | 4.5 ± 2.78 | 2–9 | |||
| Dyslipidemia | 49 | 31 (63.27%) | - | Last control | 49 | ||
| Hypertension | 49 | 37 (75.51%) | - | mean; SD | 19.3 ± 26.1 | 0–108 | |
| Diabetes Mellitus | 49 | 11 (22.45%) | - | Type of control | 49 | - | |
| Smoking | 49 | 20 (40.82%) | - | CEUS | 9 (18.37%) | - | |
| INR | 49 | TC | 24 (48.94%) | ||||
| median; IQR | 1.08 (1–1.15) | 0.92–3.25 | TC + CEUS | 10 (20.41%) | |||
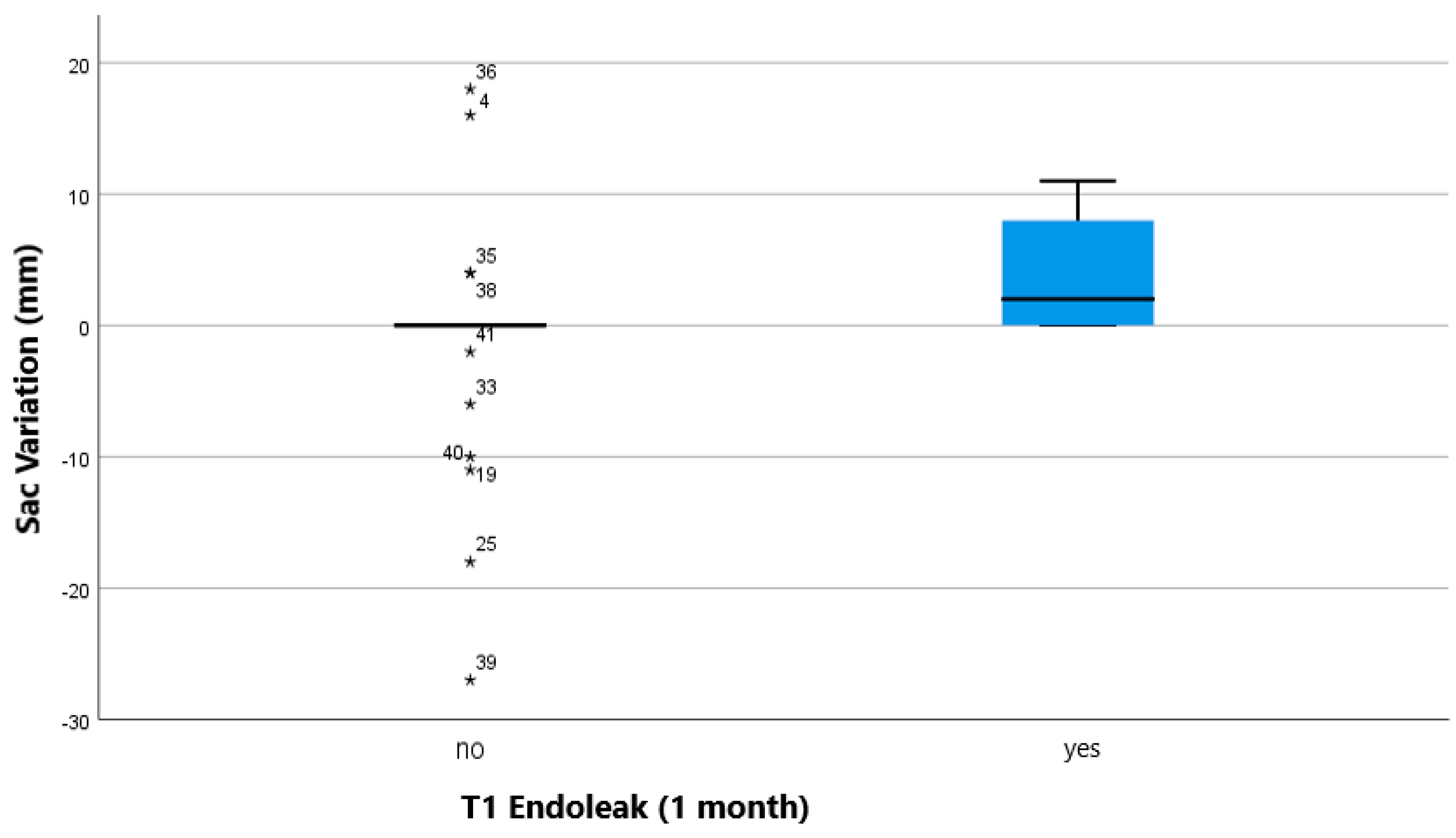
| PLT (109/L) | 49 | Sac Variation (mm) | 46 | ||||
| median; IQR | 178 (150.5–217.5) | 61–425 | median; IQR | 0 (0–2) | −27–+18 | ||
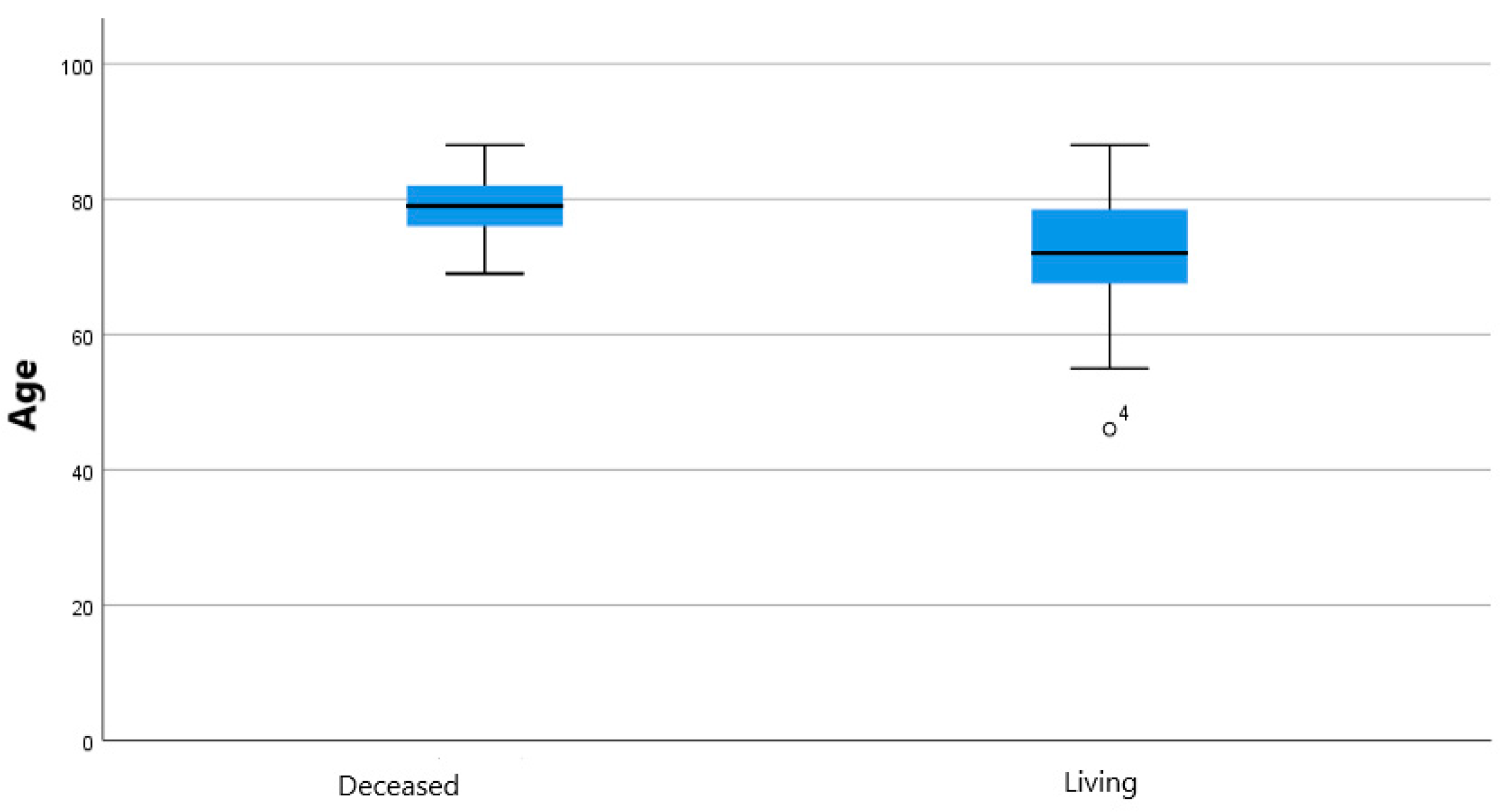
| Antiplatelets | 49 | Mortality | 48 | ||||
| no | 12 (24.49%) | deceased | 17 (35.42%) | - | |||
| yes | 29 (59.18%) | - | living | 31 (64.58%) | |||
| 2 | 8 (16.33%) | Time to exitus (months) | 17 | ||||
| Oral Bloodthinners | 49 | 5 (10.2%) | - | mean; SD | 32.55 ± 30.03 | 0.03–98.6 | |
| early EL (technical sucess) | 49 | Reintervention | 49 | 8 (16.33%) | - | ||
| no | 43 (87.75%) | - | |||||
| type 1a | 4 (8.16%) | ||||||
| type 1b | 1 (2.04%) | ||||||
| type 2 | 1 (2.04%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, F.; Coppola, A.; Ferrario, L.; De Marchi, G.; Macchi, E.; Zorzetto, G.; Franchin, M.; Piffaretti, G.; Tozzi, M.; Carcano, G.; et al. Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome. J. Clin. Med. 2022, 11, 7399. https://doi.org/10.3390/jcm11247399
Fontana F, Coppola A, Ferrario L, De Marchi G, Macchi E, Zorzetto G, Franchin M, Piffaretti G, Tozzi M, Carcano G, et al. Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome. Journal of Clinical Medicine. 2022; 11(24):7399. https://doi.org/10.3390/jcm11247399
Chicago/Turabian StyleFontana, Federico, Andrea Coppola, Lucrezia Ferrario, Giuseppe De Marchi, Edoardo Macchi, Giada Zorzetto, Marco Franchin, Gabriele Piffaretti, Matteo Tozzi, Giulio Carcano, and et al. 2022. "Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome" Journal of Clinical Medicine 11, no. 24: 7399. https://doi.org/10.3390/jcm11247399
APA StyleFontana, F., Coppola, A., Ferrario, L., De Marchi, G., Macchi, E., Zorzetto, G., Franchin, M., Piffaretti, G., Tozzi, M., Carcano, G., Piacentino, F., & Venturini, M. (2022). Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome. Journal of Clinical Medicine, 11(24), 7399. https://doi.org/10.3390/jcm11247399






