Heightened Procoagulation after Post-Operative Thromboprophylaxis Completion in Patients with Metastatic Bone Disease from Primary Colorectal Cancer
Abstract
1. Background
2. Methods
2.1. Study Participants and Design
2.2. Thrombelastography (TEG)
2.3. Platelet-Rich Plasma Preparation
2.4. Biochemical Analysis
2.5. Immunoblotting
2.6. Quantitative Proteomics
2.7. Platelet Procoagulant Membrane Dynamics (PMD) Imaging
2.8. Statistical Analysis
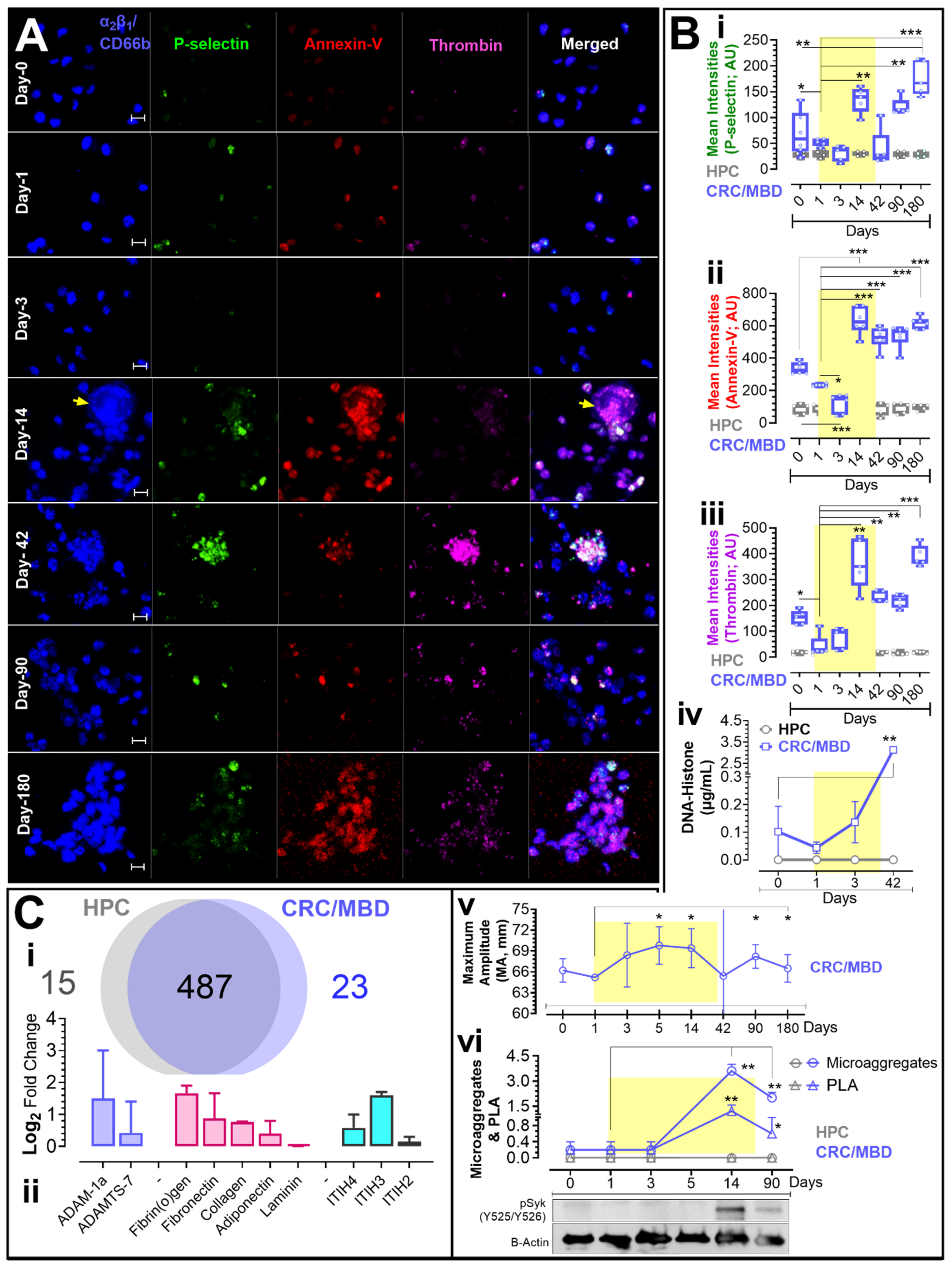
3. Results
3.1. Procoagulant Membrane Dynamics, Biochemical Analysis & Thrombelastography
3.2. Western Blot and Proteomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASH | American Society of Hematology |
| BSA | Bovine serum albumin |
| CaCl2 | Calcium chloride |
| CRC | Colorectal cancer |
| DVT | Deep vein thrombosis |
| DTT | Dithiothreitol |
| ELISA | Enzyme-linked immunosorbent assay |
| FASP | Filter-assisted separation of peptides |
| GP | Glycoprotein |
| HPC | Healthy participant controls |
| ITIH | Inter-⍺-trypsin inhibitor heavy chain proteins |
| IAA | Iodoacetamide |
| LMWH | Low molecular weight heparin |
| LEDUS | Lower extremity Doppler ultrasound |
| MBD | Metastatic bone disease |
| NETs | Neutrophil extracellular traps |
| VTE | Venous thromboembolism |
| CRC/MBD | Patients with metastatic bone disease from primary colorectal cancer |
| PS | Phosphatidylserine |
| PRP | Platelet-rich plasma |
| POD | Post-operative day |
| PMD | Procoagulant membrane dynamics |
| PE | Pulmonary embolism |
| SPE | Solid-phase extraction |
| Syk | Spleen tyrosine kinase |
| TEG | Thrombelastography |
| TCA | Trichloroacetic acid |
| PRP+ | Upper plasma-platelet fraction and buffy coat |
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, J.; Ma, Z.; Sun, R.; Seoane, J.A.; Scott Shaffer, J.; Suarez, C.J.; Berghoff, A.S.; Cremolini, C.; Falcone, A.; et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019, 51, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.S.; Fetzer, D.T.; Barron, B.J.; Joseph, U.A.; Gayed, I.W.; Wan, D.Q. Does colon cancer ever metastasize to bone first? a temporal analysis of colorectal cancer progression. BMC Cancer 2009, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
- Thrombosis Canada. Thromboprophylaxis: Orthopedic Surgery Clinical Guide. In Type of Work Clinical Guide; Thrombosis Canada: Whitby, ON, Canada, 2021. [Google Scholar]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemos. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Sciulli, M.G.; Filabozzi, P.; Tacconelli, S.; Padovano, R.; Ricciotti, E.; Capone, M.L.; Grana, M.; Carnevale, V.; Patrignani, P. Platelet activation in patients with colorectal cancer. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 79–83. [Google Scholar] [CrossRef]
- Gary, J.L.; Schneider, P.S.; Galpin, M.; Radwan, Z.; Munz, J.W.; Achor, T.S.; Prasarn, M.L.; Cotton, B.A. Can Thrombelastography Predict Venous Thromboembolic Events in Patients with Severe Extremity Trauma? J. Orthop. Trauma 2016, 30, 294–298. [Google Scholar] [CrossRef]
- Cotton, B.A.; Minei, K.M.; Radwan, Z.A.; Matijevic, N.; Pivalizza, E.; Podbielski, J.; Wade, C.E.; Kozar, R.A.; Holcomb, J.B. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J. Trauma Acute Care Surg. 2012, 72, 1470–1475, discussion 1475–1477. [Google Scholar] [CrossRef]
- Agbani, E.O.; Mahe, E.; Chaturvedi, S.; Yamaura, L.; Schneider, P.; Barber, M.R.W.; Choi, M.; Lee, A.; Skeith, L. Platelets and neutrophils co-drive procoagulant potential in secondary antiphospholipid syndrome during pregnancy. Thromb. Res. 2022, 220, 141–144. [Google Scholar] [CrossRef]
- Agbani, E.O.; Williams, C.M.; Li, Y.; van den Bosch, M.T.J.; Moore, S.F.; Mauroux, A.; Hodgson, L.; Verkman, A.S.; Hers, I.; Poole, A.W. Aquaporin-1 Regulates Platelet Procoagulant Membrane Dynamics and In Vivo Thrombosis. JCI Insight 2018, 3, e99062. [Google Scholar] [CrossRef]
- Agbani, E.O.; Van den Bosch, M.T.J.; Brown, E.; Williams, C.M.; Mattheij, N.J.A.; Cosemans, J.M.E.M.; Collins, P.W.; Heemskerk, J.W.M.; Hers, I.; Poole, A.W. Coordinated Membrane Ballooning and Procoagulant Spreading in Human Platelets. Circulation 2015, 132, 1414–1424. [Google Scholar] [CrossRef]
- De Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.-C.; Dufour, A.; Agbani, E.O. Proteomics and Metabolomics Profiling of Platelets and Plasma Mediators of Thrombo-Inflammation in Gestational Hypertension and Preeclampsia. Cells 2022, 11, 1256. [Google Scholar] [CrossRef]
- Agbani, E.O.; Williams, C.M.; Hers, I.; Poole, A.W. Membrane Ballooning in Aggregated Platelets is Synchronised and Mediates a Surge in Microvesiculation. Sci. Rep. 2017, 7, 2770. [Google Scholar] [CrossRef]
- Agbani, E.O.; Hers, I.; Poole, A.W. Temporal contribution of the platelet body and balloon to thrombin generation. Haematologica 2017, 102, e379–e381. [Google Scholar] [CrossRef][Green Version]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Pub. Co.: Reading, MD, USA, 1977. [Google Scholar]
- Agbani, E.O.; Hers, I.; Poole, A.W. Letter by Agbani et al Regarding Article, “Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface”. Arter. Thromb. Vasc. Biol. 2019, 39, e287–e289. [Google Scholar] [CrossRef]
- Thomas, G.M.; Carbo, C.; Curtis, B.R.; Martinod, K.; Mazo, I.B.; Schatzberg, D.; Cifuni, S.M.; Fuchs, T.A.; von Andrian, U.H.; Hartwig, J.H.; et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 2012, 119, 6335–6343. [Google Scholar] [CrossRef]
- Von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- McDonald, B.; Davis, R.P.; Kim, S.-J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef]
- Passacquale, G.; Ferro, A. Current concepts of platelet activation: Possibilities for therapeutic modulation of heterotypic vs. homotypic aggregation. Br. J. Clin. Pharmacol. 2011, 72, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bath, P.; Heptinstall, S. Effects of combining three different antiplatelet agents on platelets and leukocytes in whole blood in vitro. Br. J. Pharmacol. 2001, 134, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Przemyslaw, L.; Boguslaw, H.A.; Elzbieta, S.; Malgorzata, S.M. ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Rep. 2013, 46, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Watson, S.P.; Nieswandt, B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Investig. 2019, 129, 12–23. [Google Scholar] [CrossRef]
- Inoue, O.; Suzuki-Inoue, K.; Dean, W.L.; Frampton, J.; Watson, S.P. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J. Cell Biol. 2003, 160, 769–780. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Bai, X.; Liu, B.; Liu, C.J.; Xu, Q.; Zhu, Y.; Wang, N.; Kong, W.; Wang, X. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ. Res. 2009, 104, 688–698. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Gil-Pulido, J.; Sarukhanyan, E.; Burkard, P.; Shityakov, S.; Schonhart, C.; Stegner, D.; Remer, K.; Nurden, P.; Nurden, A.T.; et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 2020, 135, 1146–1160. [Google Scholar] [CrossRef]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaura, L.; Young, D.; Skeith, L.; Monument, M.J.; Jenne, C.N.; Dufour, A.; Schneider, P.; Agbani, E.O. Heightened Procoagulation after Post-Operative Thromboprophylaxis Completion in Patients with Metastatic Bone Disease from Primary Colorectal Cancer. J. Clin. Med. 2022, 11, 7397. https://doi.org/10.3390/jcm11247397
Yamaura L, Young D, Skeith L, Monument MJ, Jenne CN, Dufour A, Schneider P, Agbani EO. Heightened Procoagulation after Post-Operative Thromboprophylaxis Completion in Patients with Metastatic Bone Disease from Primary Colorectal Cancer. Journal of Clinical Medicine. 2022; 11(24):7397. https://doi.org/10.3390/jcm11247397
Chicago/Turabian StyleYamaura, Lisa, Daniel Young, Leslie Skeith, Michael J. Monument, Craig N. Jenne, Antoine Dufour, Prism Schneider, and Ejaife O. Agbani. 2022. "Heightened Procoagulation after Post-Operative Thromboprophylaxis Completion in Patients with Metastatic Bone Disease from Primary Colorectal Cancer" Journal of Clinical Medicine 11, no. 24: 7397. https://doi.org/10.3390/jcm11247397
APA StyleYamaura, L., Young, D., Skeith, L., Monument, M. J., Jenne, C. N., Dufour, A., Schneider, P., & Agbani, E. O. (2022). Heightened Procoagulation after Post-Operative Thromboprophylaxis Completion in Patients with Metastatic Bone Disease from Primary Colorectal Cancer. Journal of Clinical Medicine, 11(24), 7397. https://doi.org/10.3390/jcm11247397





