Thrombotic Events during Lenvatinib Treatment: A Single Institution Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
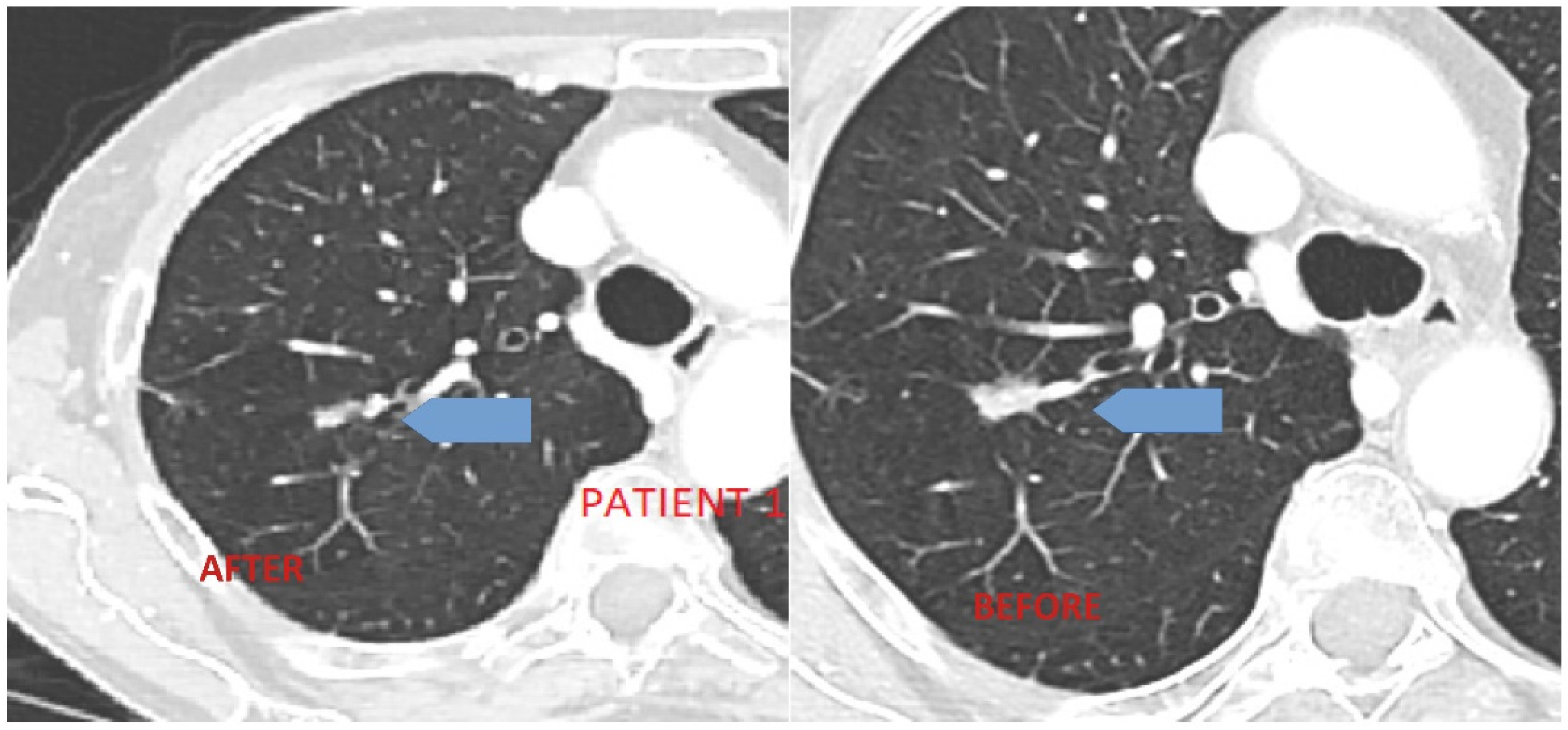
3.1. Case 1
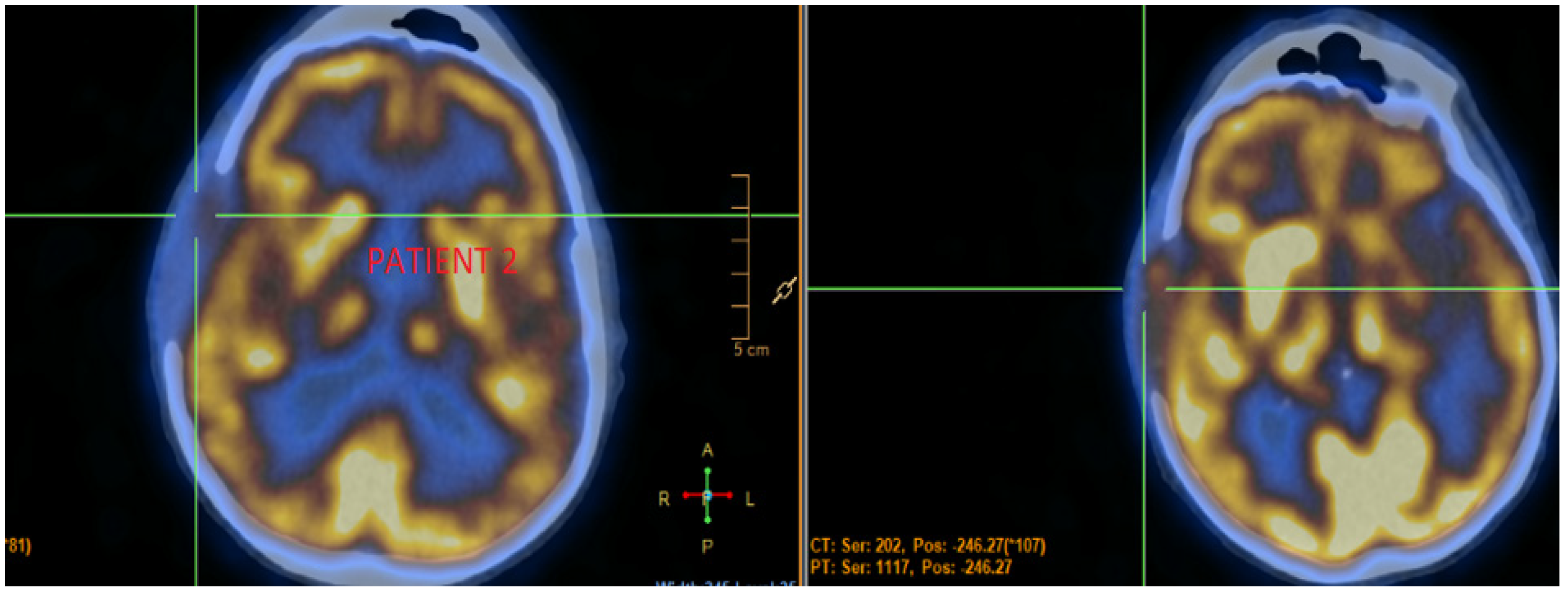
3.2. Case 2
3.3. Case 3
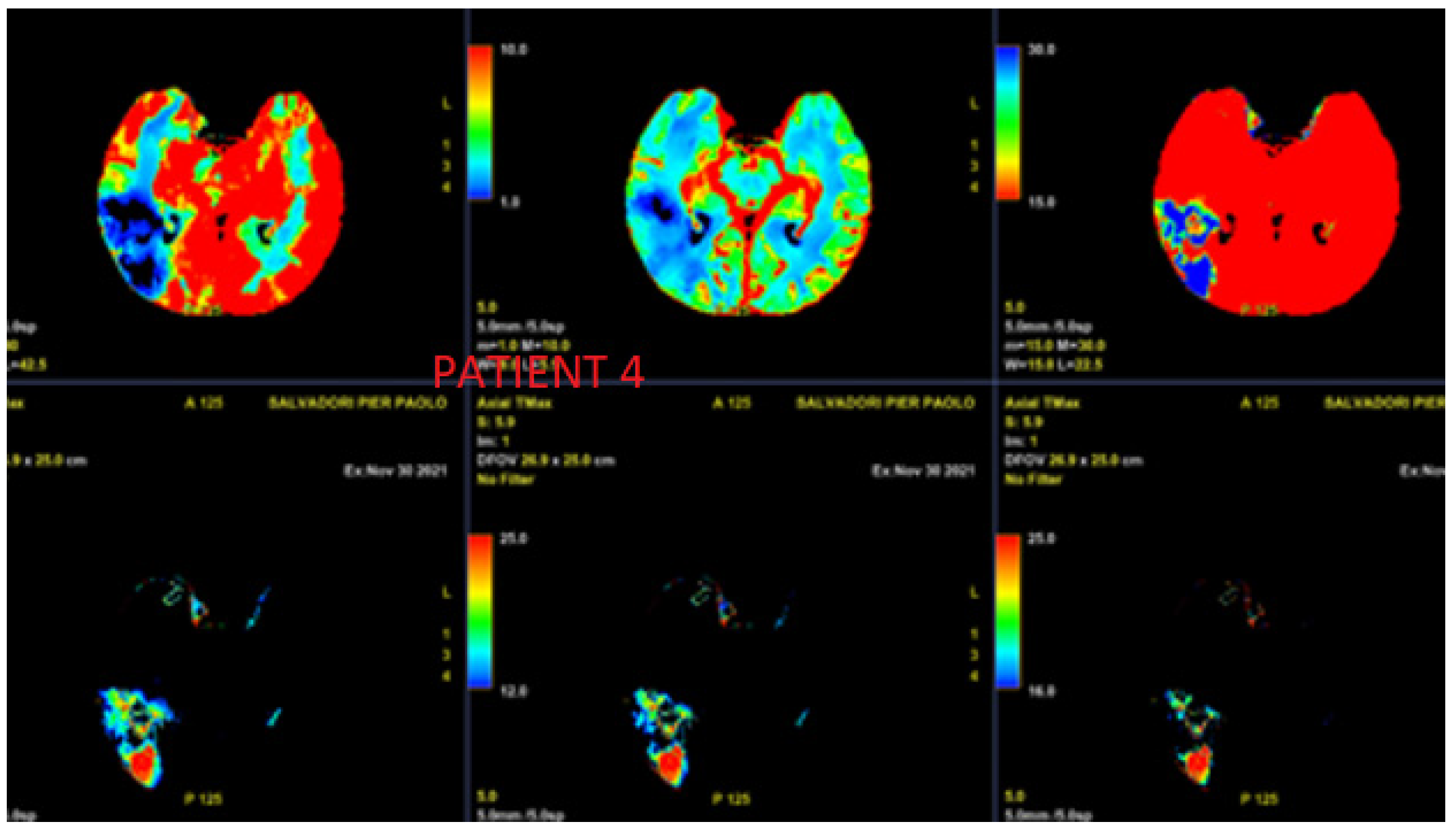
3.4. Case 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, L.A.; Kuo, J.H. Advancements in the treatment of differentiated thyroid cancer. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211000251. [Google Scholar] [CrossRef]
- I Numeri Del Cancro in Italia: Tiroide (Locati L Bernardo G). Available online: https://www.aiom.it/wp-content/uploads/2021/10/2021_NumeriCancro_web.pdf (accessed on 2 December 2022).
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Geiger, J.L.; ISmaila, N.; Beadle, B.; Caudell, J.J.; CHau, N.; Deschler, D.; Glasonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Berg, E.; Adam, P.; Weber, G.L.; Pfestroff, A.; Luster, M.; Kutsch, J.M.; Lapa, C.; Sandner, B.; Rayes, N.; et al. Real-World Efficacy and Safety of Multi-Tyrosine Kinase Inhibitors in Radioiodine Refractory Thyroid Cancer. Thyroid 2021, 31, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Boucher, A.; Lemieux, B.; Rondeau, G.; Lebœuf, R.; Ste-Marie, L.G.; Le, X.K.; Mircescu, H. Lenvatinib Therapy for Advanced Thyroid Cancer: Real-Life Data on Safety, Efficacy, and Some Rare Side Effects. J. Endocr. Soc. 2022, 6, bvac048. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tahara, M.; Ito, K.; Tori, M.; Kiyota, N.; Yoshida, K.; Sakata, Y.; Yoshida, A. Safety and Effectiveness of Lenvatinib in 594 Patients with Unresectable Thyroid Cancer in an All-Case Post-Marketing Observational Study in Japan. Adv. Ther. 2020, 37, 3850–3862. [Google Scholar] [CrossRef] [PubMed]
- Rendl, G.; Sipos, B.; Becherer, A.; Sorko, S.; Trummer, C.; Raderer, M.; Hitzl, W.; Ardelt, M.; Gallowitsch, H.J.; Pirich, C. Real-World Data for Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer (RELEVANT): A Retrospective Multicentric Analysis of Clinical Practice in Austria. Int. J. Endocrinol. 2020, 2020, 8834148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.-M.; Chang, H.; Kim, B.-W.; Lee, Y.S.; Chang, H.-S.; Park, C.S. Safety of Tyrosine Kinase Inhibitors in Patients With Differentiated Thyroid Cancer: Real-World Use of Lenvatinib and Sorafenib in Korea. Front. Endocrinol. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Piovesan, A.; Durante, C.; Bregni, M.; Castagna, M.G.; Zovato, S.; Giusti, M.; Ibrahim, T.; Puxeddu, E.; Fedele, G.; et al. Real-world efficacy and safety of Lenvatinib: Data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur. J. Cancer 2019, 118, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-X.; Shen, Z.; Tang, L.-N.; Yao, Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: An up-to-date meta-analysis. Crit. Rev. Oncol. 2014, 92, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Takahashib, S. Managing the adverse events associated with Lenv therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 2019, 46, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, J.Y.; Li, J.; Zhang, B.; Liu, Y.; Zhang, B.; Jing, J. Risk of venous and arterial thromboembolic events associated with tyrosine kinase inhibitors in advanced thyroid cancer: A meta-analysis and systematic review. Oncotarget 2018, 10, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Tran, C.; Baxi, S.; Katabi, N.; Antonescu, C.R.; Ostrovnaya, I.; Haque, S.S.; Pfister, D.G.; et al. Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2019, 37, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Galbiati, D.; Calareso, G.; Alfieri, S.; Singer, S.; Cavalieri, S.; Bergamini, C.; Bossi, P.; Orlandi, E.; Resteghini, C.; et al. Patients with adenoid cystic carcinomas of the salivary glands treated with Lenvv: Activity and quality of life. Cancer 2020, 126, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Platini, F.; Cavalieri, S.; Alfieri, S.; Bergamini, C.; Resteghini, C.; Bottiglieri, A.; Colombo, E.; Mazzeo, L.; Licitra, L.; Paolini, B.; et al. Late toxicities burden in patients with radioiodine-refractory differentiated thyroid cancer treated with Lenvv. Endocrine 2021, 73, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Panaseykin, Y.; Konda, B.; de la Fouchardiere, C.; Hughes, B.G.M.; Gianoukakis, A.G.; Joo Park, Y.; Romanov, I.; Krzyzanowska, M.K.; Leboulleux, S.; et al. A Randomized Study of Lenvatinib 18 mg vs. 24 mg in Patients with Radioiodine-Refractory Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2022, 107, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Schutz, F.A.; Je, Y.; Richards, C.J.; Choueiri, T.K. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in pts with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J. Clin. Oncol. 2012, 30, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, S.; Murayama, D.; Kato, S.; Matsui, A. Relationship between adverse events associated with Lenv treatment for thyroid cancer and pt prognosis. Mol. Clin. Oncol. 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]

| Pts | Age | Histology | Sex | Smoking History | High Cholesterol | High Blood Pressure | BMI | Start Lenv | End Lenv | TE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 72 | Fo | F | N | N | Y | 17.2 | 23 July 2015 | 30 November 2021 | 30 November 2021 |
| 2 | 71 | Pa | M | Y | Y | Y | 20.6 | 22 May 2015 | 2 July 2021 | |
| 3 | 89 | Pa | M | N | N | Y | 21 | 20 February 2017 | 25 October 2017 | |
| 4 | 71 | Pa | F | Y | N | Y | 28 | 10 July 2017 | 27 December 2017 | |
| 5 * | 75 | Pa | M | Y | N | Y | 21.9 | 26 March 2019 | 30 December 2020 | 30 December 2020 |
| 6 | 84 | Pa | M | Y | N | Y | 23 | 5 February 2018 | 31 December 2020 | |
| 7 | 54 | Pa | F | Y | N | Y | 17 | 26 April 2021 | 31 October 2021 | |
| 8 * | 56 | ACC | M | Y | N | Y | 24.16 | 21 September 2021 | 30 November 2021 | 30 November 2021 |
| 9 * | 70 | Pa | F | N | N | Y | 20.2 | 7 February 2021 | 17 March 2021 | 17 March 2021 |
| 10 | 65 | Pa | F | Y | N | N | 21 | 10 May 2021 | ongoing | |
| 11 | 59 | Pa | F | N | N | N | 18.9 | 9 August 2021 | ongoing | |
| 12 | 59 | Pa | M | Y | N | N | 22 | 16 August 2021 | ongoing | |
| 13 | 75 | Pa | F | Y | Y | N | 24.5 | 22 November 2021 | ongoing | |
| 14 | 40 | Fo | M | Y | N | N | 26.1 | 15 November 2021 | ongoing | |
| 15 | 73 | Pa | F | N | Y | N | 20.3 | 6 December 2021 | ongoing | |
| 16 | 78 | Fo | M | Y | N | N | 22.1 | 23 July 2015 | ongoing |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sex | F | M | F | M |
| Age | 72 | 75 | 70 | 56 |
| Previous surgery | 3 | 2 | 2 | 2 |
| Lenv start | July 2015 | March 2019 | Feb 2021 | Sep 2021 |
| Reduction dose level | 10 mg | 10 mg | 20 mg | 14 mg |
| TE event | Nov 2020 | Jan 2021 | March 2021 | Nov 2021 |
| High cholesterol level | No | No | No | No |
| Diabetes | No | No | No | No |
| Smoking | Yes | Yes | Yes | Yes |
| Atrial fibrillation | No | No | No | No |
| Overweight | No | No | No | No |
| Sedentary | No | No | No | No |
| High blood pressure | Yes | Yes | Yes | Yes |
| ECOG PS 0-1 | Yes | Yes | No | Yes |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| WBC baseline | 6.317 × 109/L | 8.560 × 109/L | 5.600 × 109/L | 6.520 × 109/L |
| WBC at the event | 8.15 × 109/L | 1.31 × 109/L | 6.8 × 109/L | 6.85 × 109/L |
| RBC baseline | 5.4 × 1012/L | 4.5 × 1012/L | 3.52 × 1012/L | 4.2 × 1012/L |
| RBC at the event | 5.5 × 1012/L | 3.8 × 1012/L | 4.1 × 1012/L | 4.3 × 1012/L |
| PLT baseline | 229 × 109/L | 225 × 109/L | 315 × 109/L | 258 × 109/L |
| PLT at the event | 214 × 109/L | 187 × 109/L | 250 × 109/L | 195 × 109/L |
| Symptom | G1-2 | G3-4 | SELECT G3-4 | Italian Locati 2019 | Relevant Austrian Rendl 2020 | Taiwan Jiang 2021 | Canadian Hamidi 2022 | Korea Kim 2019 | Japanese Takahashi 2020 |
|---|---|---|---|---|---|---|---|---|---|
| Hypertension | 66 | 25 | 41.1 | 4.7 | 26 | 15.4–30.8 | 59.3 | 73.9 | 60.2 |
| Decreased appetite | 40 | 6.25 | 5.4 | 3.0 | 7 | 0–7.7 | 11.1 | 4.3 | 4.5 |
| Decreased weight | 50 | 6.25 | 9.6 | 4.5 | 7 | 0–7.7 | 14.8 | 4.3 | 4.5 |
| Oropharyngeal pain | 6.25 | NA | NA | NA | NA | NA | NA | NA | |
| Palmar-plantar dysesthesia syndrome | 75 | 12.5 | 3.4 | 1.9 | 2 | 30.8–15.4 | 7.4 | 30.4 | 5.8 |
| Arthralgia | 26 | 0 | 0 | NR | NR | NR | 0 | NR | NR |
| Dysphonia | 36 | 6.25 | 1 | NR | NR | NR | 0 | NR | NR |
| TSH changes | 36 | 6.25 | NR | NR | NR | NR | 25.9 | NR | |
| Diarrhoea | 56 | 12.5 | 4.8 | 2.8 | 7 | NR | 11.1 | 4.3 | 3.8 |
| Fatigue | 64 | 12.5 | 9.2 | 8.2 | 0 | 23.1–7.7 | 0 | NR | 2.3 |
| Proteinuria | 0 | 7.7–53.8 | 7.4 | 8.7 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denaro, N.; Garrone, O.; Ghidini, M.; Tomasello, G.; Hahne, J.C.; Merlano, M.C.; Locati, L.D. Thrombotic Events during Lenvatinib Treatment: A Single Institution Experience. J. Clin. Med. 2022, 11, 7312. https://doi.org/10.3390/jcm11247312
Denaro N, Garrone O, Ghidini M, Tomasello G, Hahne JC, Merlano MC, Locati LD. Thrombotic Events during Lenvatinib Treatment: A Single Institution Experience. Journal of Clinical Medicine. 2022; 11(24):7312. https://doi.org/10.3390/jcm11247312
Chicago/Turabian StyleDenaro, Nerina, Ornella Garrone, Michele Ghidini, Gianluca Tomasello, Jens Claus Hahne, Marco Carlo Merlano, and Laura Deborah Locati. 2022. "Thrombotic Events during Lenvatinib Treatment: A Single Institution Experience" Journal of Clinical Medicine 11, no. 24: 7312. https://doi.org/10.3390/jcm11247312
APA StyleDenaro, N., Garrone, O., Ghidini, M., Tomasello, G., Hahne, J. C., Merlano, M. C., & Locati, L. D. (2022). Thrombotic Events during Lenvatinib Treatment: A Single Institution Experience. Journal of Clinical Medicine, 11(24), 7312. https://doi.org/10.3390/jcm11247312









