Abstract
Late-onset asthma (LOA) differs from early-onset asthma (EOA) in terms of prognosis and the treatment response because it has a much worse prognosis and a poorer response to standard asthma treatment. This study sought to investigate the characteristics and clinical outcomes of asthma patients with phenotypes distinguished by age at onset and atopy status. We prospectively recruited patients with asthma who were registered in a pay-for-performance program operated by Taiwan’s National Health Insurance Administration (NHIA). These patients received regular outpatient treatment for at least 1 year at every outpatient clinic visit since 2019. Baseline characteristics and clinical outcomes were compared between patients with LOA (≥40 years) and those with EOA (<40 years). Of the consecutive 101 patients with asthma, 21 patients (20.7%) had EOA and 80 (79.3%) had LOA. In the 12-month period, patients with EOA had higher declines in forced expiratory volume in one second (FEV1; −2.1 ± 8.4 vs. 6.8 ± 13.1, % of predicted value, p = 0.037) and forced vital capacity (FVC; −4.6 ± 12.0 vs. 6.1 ± 13.6, % of predicted value, p = 0.023) than patients with LOA. Patients with nonatopic EOA had a significantly higher exacerbation rate at 12 months than patients with nonatopic LOA (50% vs. 11.8%, p = 0.012). Identification of different phenotypes of asthma is important in clinical practice because treatment responses may differ.
1. Introduction
Asthma is a heterogeneous disease with multiple phenotypes depending on the severity of symptoms, extent of airflow limitation, level of asthma control, frequency of exacerbations, type of underlying airway inflammation (eosinophilic or non-eosinophilic), and age at asthma onset [1]. The onset of asthma is often assumed to occur in childhood or early adulthood, but 30–40% of adults with asthma experience their initial symptoms after the age of 40 years. In addition, up to 52% of asthmatic patients have their “first asthma attack” after the age of 40 years [2,3]. Late-onset asthma (LOA) has a worse prognosis and a poorer response to standard asthma treatment compared with early-onset asthma (EOA) [4]. EOA and LOA are the most common phenotypes of asthma [5], and recent cluster-based studies have also identified age at onset as a key factor in distinguishing asthma phenotypes [6]. Atopic asthma represents the most common form of asthma in children [7], and it is often characterized by eosinophils and T helper 2 (Th2) cell-driven infiltration of the bronchial mucosa, circulating specific immunoglobulin E (IgE) antibodies, positive skin tests for common aeroallergens, and airway hyperresponsiveness [8]. Nonatopic asthmatics are significantly older than atopic asthmatics [8]. The etiologies of these phenotypes, including airway inflammation, natural progression, and responsiveness to treatment, are believed to differ [6]. According to a previous study, patients with nonatopic LOA are at a higher risk of developing persistent airflow limitation than those with atopy [9]. In contrast to patients with atopic EOA, patients with nonatopic EOA respond poorly to inhaled corticosteroids [10]. Identifying and evaluating these phenotypes will provide a comprehensive understanding of the causes and progression of asthma in adults, and this may facilitate the development of more targeted preventive strategies and treatment alternatives.
The clinical outcomes and response to treatment in asthma patients with different phenotypes of asthma and atopic status have been investigated. However, most of the studies are cross-sectional or population-based, focusing on the characteristics of different phenotypes in asthmatics. Therefore, collecting real-world data through longitudinal assessments of these phenotypes may provide a comprehensive understanding of clinical outcomes, such as the frequency of exacerbations and pulmonary function decline. However, the reliability of the results is influenced mainly by whether treatment management and patient compliance are standardized. The pay-for-performance program has been implemented by the National Health Insurance Administration (NHIA) in Taiwan since 2001 to strengthen the management and health education of asthma patients [11]. Furthermore, the NHIA urges certified physicians and case managers to provide in-person instructions on asthma control, asthma care planning, and proper inhaler use [12], and the outcomes are regularly monitored by the NHIA. Because of this, we could follow up patients throughout the study period and ensure the consistency of their treatment plans. This study sought to investigate the characteristics and clinical outcomes of asthma patients with phenotypes distinguished by age on onset and atopy status.
2. Materials and Methods
2.1. Study Design and Patient Recruitment
This was a prospective cohort study of asthma patients participating in a pay-for-performance program, who were recruited consecutively at Chang Gung Memorial Hospital, Linkou Branch. Patients were diagnosed with asthma by a pulmonologist on the basis of the concordance of respiratory symptoms, pulmonary function, and responsiveness to asthma treatment. After receiving their confirmed asthma diagnosis from a physician, they were followed up every 3 months for at least 1 year. The pay-for-performance program is implemented by the NHIA in Taiwan to strengthen the management and health education of asthma patients. For sample recruitment, patients who were registered in the pay-for-performance program and had a major diagnosis code (ICD-10 code J45) present at least twice within 90 days were consecutively enrolled into our study after providing their written informed consent. Patients with incomplete pulmonary function data, noncompliance with regular clinic visits, and coexisting chronic obstructive pulmonary disease (COPD) were excluded. The definition of adequate compliance was patients with adequate medication compliance by self-report of more than 80% use of prescribed medications and regular clinical visits. The pay-for-performance program was implemented in qualified medical centers in Taiwan by NHIA. This study recruited patients who were diagnosed as having asthma and received at least 2 clinic visits within 90 days in our hospital. Enrollment in the program was voluntary for patients, and they signed the patient agreement consent. The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital (No.201900211B0).
2.2. Patient Data
In this study, the following data were collected from patients on the basis of questionnaires or medical records: age at asthma diagnosis by a physician, history of pediatric dyspnea, frequency of bronchitis, gender, family history of asthma, smoking status, exacerbations in the previous year, and pulmonary function and T2 inflammation biomarkers, such as eosinophil count, eosinophil cation protein (ECP), total immunoglobulin E (IgE) level, and specific IgE (ImmunoCAP, Phadia, Uppsala, Sweden). Patients with any positive specific IgE to allergens (>0.35 kUA/L) were considered atopic [12]. We also recorded the number of acute asthma exacerbations, including hospitalization, emergency room visits, and systemic corticosteroid bursts, using medical records and patients’ self-reported data. The level of asthma control was evaluated using the asthma control test (ACT, a 5-question questionnaire, with each question scored from 1 to 5. The lower summed scores for all questions indicate poorer asthma control). Patients with LOA were defined as those who were ≥40 years old at the time of asthma onset without a history of pediatric dyspnea or bronchitis. Otherwise, they were classified as having EOA [1].
2.3. Outcome Measurement
A total of 101 patients were divided into 2 groups on the basis of the age at asthma onset. In each group, patients were further classified as having atopic and nonatopic asthma. Then, the clinical outcomes of each group at 1 year were compared. We performed spirometry in accordance with the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines [13], recording forced expiratory volume in 1 s (FEV1) (% predicted), forced vital capacity (FVC), and FEV1/FVC. We also determined the number of asthma exacerbations (defined as a decline in FEV1 to <60% of the personal best, requiring an OCS burst and an unscheduled doctor/emergency room visit or hospitalization) and asthma-related hospitalizations during the 12-month period prior to recruitment and during the 12-month observation period.
2.4. Statistical Analysis
Unless otherwise indicated, all data are expressed as mean ± standard deviation or percentages. The Student’s t-test was used to compare the means of normally distributed continuous data between the 2 groups; otherwise, the Mann–Whitney test was used. The chi-squared test or Fisher’s exact test was used to examine the correlations between categorical variables. Statistical analyses were performed using SPSS software, version 26 (IBM Corporation, Armonk, NY, USA). The statistical significance level was set at a p-value of <0.05.
3. Results
3.1. Characteristics of Patients with EOA Compared with Patients with LOA
Patients in the pay-for-performance program were screened from April 2019 to December 2021. A total of 145 adult patients with asthma were enrolled. We excluded 16 patients because of missing regular asthma clinic visits, 17 patients because of missing spirometer measurements, and 11 patients because they had coexisting COPD. The remaining 101 asthma patients were eligible for analysis. Figure 1 provides the study flowchart. Among these asthmatics, 21 patients (20.7%) had EOA. The characteristics of patients with EOA and LOA are shown in Table 1. The average onset age of patients with EOA and LOA was 23.4 and 61.8 years, respectively. Patients with EOA were younger, had a longer duration of asthma, had a higher rate of family history of asthma, and had more comorbidities with allergic rhinitis or rhinosinusitis with or without polyps compared with patients with LOA. No difference was observed between the two groups in terms of gender, smoking habits, weight status, exacerbations in the last year, medications, or ACT score. The pre-bronchodilator FVC and FEV1 were higher in patients with EOA than in those with LOA. Regarding allergic status, the proportion of atopy was higher in the EOA group than in the LOA group.
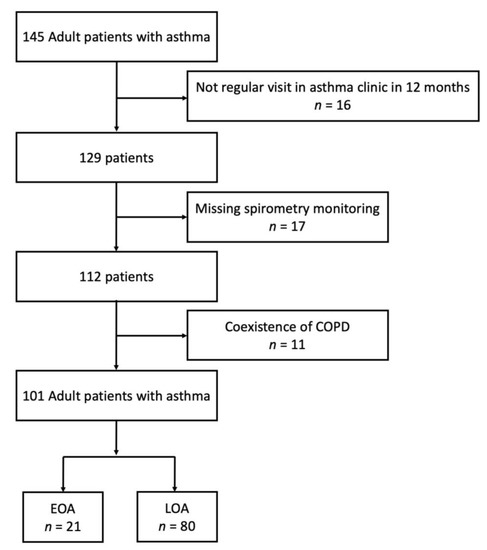
Figure 1.
Study profile: The number of patients enrolled and analyzed in the study.

Table 1.
Characteristics of patients with early-onset and late-onset asthma.
The characteristics of patients with atopic and non-atopic status are shown in Table 1. Atopic patients were younger, had a lower BMI, had a longer duration of asthma, had a lower rate of male gender, and had more comorbidities with allergic rhinitis or obstructive sleep apnea compared with non-atopic patients. No difference was observed between the two groups in terms of smoking habits, family history of asthma, exacerbations in the last year, medications, or ACT score. The pre-bronchodilator FVC was higher in atopic patients than in non-atopic patients. Regarding allergic status, the IgE levels and rate of fungus sensitization were higher in the atopic group than in the non-atopic group.
3.2. Clinical Outcomes after 12-Month Treatment
Table 2 shows the pulmonary function at 12 months for patients with EOA and LOA. The mean difference in the percentage of predicted FVC and FEV1 from baseline declined in the EOA group, whereas it increased in the LOA group. Patients with EOA had greater declines in FEV1 (−2.1 ± 8.4 vs. 6.8 ± 13.1, % of the predicted value, p = 0.037) and FVC (−4.6 ± 12.0 vs. 6.1 ± 13.6, % of the predicted value, p = 0.023) than patients with LOA over a 12-month period (Figure 2). Both groups had similar baseline exacerbation rates. No difference was noted between the groups in clinical outcomes at 12 months. In the total asthma cohort (Figure 3), the incidence of total acute exacerbation events (28.7% vs. 12.9%, p = 0.009), emergency room visits (12.9% vs. 1.0%, p = 0.001), and hospitalizations (11.9% vs. 0%, p = 0.0003) decreased after 12 months of treatment. In the subgroups of atopic asthma patients, the incidence of AE decreased in both the EOA (20% (3/15) vs. 6.7% (1/15)) and LOA (29.4% (10/34) vs. 11.8% (3/34)) groups after 12-month treatment (Figure 4). In the subgroups of nonatopic asthma patients, the incidence of AE decreased in the LOA (32.4% (14/46) vs. 10.9% (5/46)) group after 12-month treatment (Figure 4). However, the incidence of AE increased in the EOA (33.3% (2/6) vs. 50% (3/6)) group after 12-month treatment. In the nonatopic subgroups, patients with EOA had a significantly higher exacerbation rate at 12 months than those with LOA (50% vs. 11.8%, p = 0.012). In contrast, the pulmonary function and acute exacerbation at 12 months were similar in the atopic and non-atopic groups (Table 2).

Table 2.
Clinical outcomes of patients with asthma after 12-month treatment.
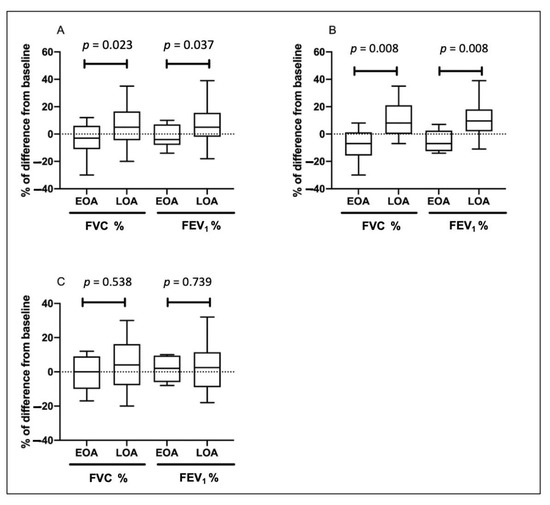
Figure 2.
Change of forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) in patients with early-onset asthma (EOA) and late-onset asthma (LOA) after 12-month treatment in the total cohort (A), patients <65 years old (B), and patients ≥65 years old (C). Boxplots show the median (bar), the first and third quartiles (box), and the 1st and 99th percentiles (whiskers) of the biomarkers level for each asthma status.
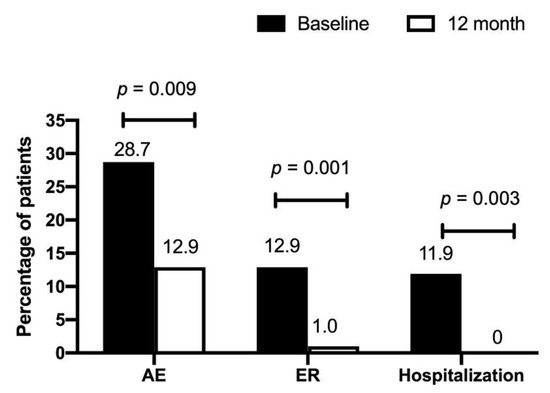
Figure 3.
Percentage of acute exacerbation (AE), emergency room (ER) visits, and hospitalizations in patients with asthma at baseline and after 12-month treatment.
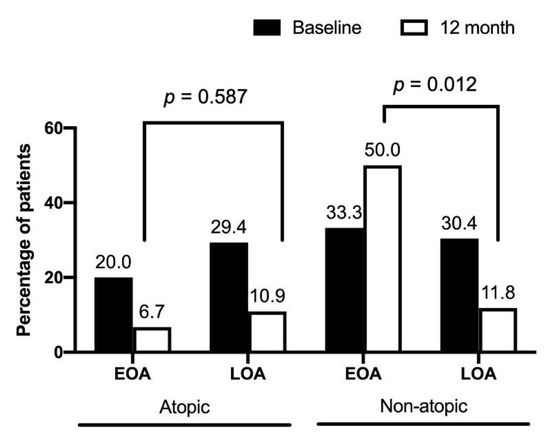
Figure 4.
Percentage of acute exacerbations in early-onset asthma (EOA) and late-onset asthma (LOA) patients with atopic and nonatopic status after 12-month treatment. The baseline percentages of acute exacerbation were similar between EOA and LOA in patients with atopic and non-atopic status.
To elucidate the impact of age on clinical outcomes of patients with EOA and LOA, patients were stratified as age < and ≥ 65 years old. Among patients <65 years old (n = 43), there were 15 EOA patients and 28 LOA patients. The characteristics of patients with EOA and LOA are listed in Supplementary Table S1. In the subgroup of patients ≥65 years old, 6 patients were EOA and 52 patients were LOA. The baseline characteristics of patients ≥65 years old are shown in Table S3. In patients <65 years old, EOA patients had greater FEV1 decline than LOA patients over a 12-month period (−5.3 ± 8.0 vs. 10.2 ± 12.3, % of the predicted value, p = 0.008) and FVC (−8.0 ± 12.7 vs. 8.4 ± 11.9, % of the predicted value, p = 0.008) (Table S2; Figure 2B). In patients ≥65 years old, the changes of pulmonary function at 12 months were similar in EOA and LOA groups (Table S4; Figure 2C).
3.3. The Treatments for Asthma in Each Group
Table 3 shows the treatment for patients with atopic and non-atopic status. In atopic patients, there was no difference in treatment for asthma in EOA and LOA groups. In non-atopic patients, there were more EOA patients who received OCS and biologics treatment than LOA patients.

Table 3.
The treatment for patients with asthma.
4. Discussion
Our study results revealed that patients with EOA were younger, had a longer asthma duration, and had increased rates of allergic rhinitis, rhinosinusitis with or without polyps, and atopy than patients with LOA [14]. After 12 months of treatment, the rates of acute exacerbation events, including hospitalizations, emergency room visits, and systemic corticosteroid bursts, decreased in the total asthma cohort. The EOA group showed a greater decline in pulmonary function than the LOA group. Considering the atopic and asthma onset features, all three subgroups had a decreased incidence of AE, except that patients with nonatopic EOA had an increased incidence of exacerbations after 12 months of treatment.
This study found that after 12-month treatment, patients with EOA had an increased pulmonary function decline compared with patients with LOA. However, most previous studies have reported that patients with LOA exhibit a rapid decline in pulmonary function [9,15]. The possible reasons for the conflicting results regarding pulmonary function decline in patients with EOA may be due to the duration of asthma. In the present study, patients with EOA had a longer duration of asthma than patients with LOA. Long-lasting chronic airway inflammation may adversely affect pulmonary function [16,17]. In addition, the prevalence of airway remodeling appears to be higher in patients with EOA than in patients with LOA. The prominent presence of airway remodeling may inversely affect pulmonary function [18].
This study revealed a significant decrease in AE events after the 12-month asthma treatment in the total study cohort. Patients with EOA and LOA had similar responses to 12-month treatment in terms of the frequency of exacerbations. This may be due to high compliance and regular outpatient clinic follow-up visits. Our study results also showed that patients with nonatopic EOA had an increased frequency of exacerbations after 12-month treatment. Typically, EOA is highly associated with T2-high phenotypes, such as high eosinophilic and atopic features. However, a subset of patients with EOA exhibit nonatopic phenotypes [10]. Previous studies have reported that patients with nonatopic EOA respond poorly to inhaled corticosteroids compared with those with atopic asthma [10], suggesting that nonatopic asthma has various predisposing factors and causal mechanisms, which are possibly related to infections caused by viruses, atypical bacteria, or fungi [9,19]. Recent evidence has also demonstrated that patients with nonatopic asthma typically have more severe symptoms and require higher doses of inhaled corticosteroids for controlling symptoms [20]. In addition, our results demonstrated that in non-atopic patients, there were more EOA patients who received OCS and biologics treatment than LOA patients. The results indicate that non-atopic EOA patients may have more severe disease course and require OCS and biologics treatment than do non-atopic LOA patients. Therefore, further study is necessary to investigate the treatment response of patients with nonatopic EOA who receive standard treatment for asthma.
There is an increasing awareness of the heterogeneous features of asthma and treatment responsiveness. Given that pulmonary function decline is one of the risk factors for asthma, early identification of patients at high risk of accelerated decline in pulmonary function is important because irreversible airflow obstruction is associated with increased morbidity and mortality [21,22].
Our study has several limitations. First, the sample size was small because we included only patients who had high compliance and had regular outpatient follow-up visits for at least 1 year. The possible selection bias may occur due to recruitment of only compliant patients. Therefore, use of these results in asthma patients should be applied with caution. Second, no fractional exhaled nitric oxide (FeNO) or biomarker data were collected in our study. Third, the assessment of acute exacerbations in the previous year was based on self-reported information; thus, it could be influenced by recall bias.
It is important to distinguish between different phenotypes of asthma because treatment responses may vary. A significant proportion of patients with EOA lack the characteristics of atopy and may have an increased incidence of acute exacerbations, even under the standard treatment for asthma. Overall, identifying these phenotypes will improve prognosis and treatment guidance. Therefore, future research should focus on identifying the inflammatory pathways in nonatopic asthma and potential phenotype-guided therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247309/s1. Table S1. Characteristics of patients (<65 years old) with early-onset and late-onset asthma. Table S2. Clinical outcomes of patients (<65 years old) with asthma after 12-month treatment. Table S3. Characteristics of patients (≥65 years old) with early-onset and late-onset asthma. Table S4. Clinical outcomes of patients (≥65 years old) with asthma after 12-month treatment.
Author Contributions
Conceptualization, P.-J.C. and C.-Y.L.; methodology, B.-C.W., C.-H.C., Y.-C.T., T.-Y.L., P.-J.C., C.-Y.L. and S.-M.L.; formal analysis, B.-C.W., C.-H.C., Y.-C.T., T.-Y.L., P.-J.C., C.-Y.L. and S.-M.L.; investigation, B.-C.W., C.-H.C., Y.-C.T., T.-Y.L., P.-J.C., C.-Y.L. and S.-M.L.; resources, B.-C.W., C.-H.C., Y.-C.T., T.-Y.L., P.-J.C., C.-Y.L. and S.-M.L.; data curation, B.-C.W., C.-H.C. and Y.-C.T.; writing—original draft preparation, B.-C.W., T.-Y.L. and S.-M.L.; writing—review and editing, B.-C.W., T.-Y.L. and S.-M.L.; supervision, S.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chang Gung Medical Research Projects (CIRPG3K2011) and the Ministry of Science and Technology, Taiwan (MOST 111-2321-B-182-001).
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Chang Gung Memorial Foundation (IRB No. 202100712B0).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brusselle, G.; Germinaro, M.; Weiss, S.; Zangrilli, J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm. Pharmacol. Ther. 2017, 43, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Qualls, C.; Schuyler, M.; Arynchyn, A.; Alvarado, J.H.; Smith, L.J.; Jacobs, D.R., Jr. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. The longitudinal CARDIA study. Ann. Am. Thorac. Soc. 2013, 10, 188–197. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, S.B.; Venekamp, L.N.; Bel, E.H. Adult-onset asthma: Is it really different? Eur. Respir. Rev. 2013, 22, 44–52. [Google Scholar] [CrossRef]
- Quirce, S.; Heffler, E.; Nenasheva, N.; Demoly, P.; Menzies-Gow, A.; Moreira-Jorge, A.; Nissen, F.; Hanania, N.A. Revisiting late-onset asthma: Clinical characteristics and association with allergy. J. Asthma Allergy 2020, 13, 743. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Anderson, H.; Pottier, A.; Strachan, D. Asthma from birth to age 23: Incidence and relation to prior and concurrent atopic disease. Thorax 1992, 47, 537–542. [Google Scholar] [CrossRef]
- Humbert, M.; Menz, G.; Ying, S.; Corrigan, C.J.; Robinson, D.S.; Durham, S.R.; Kay, A.B. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: More similarities than differences. Immunol. Today 1999, 20, 528–533. [Google Scholar] [CrossRef]
- Amelink, M.; de Nijs, S.B.; Berger, M.; Weersink, E.J.; ten Brinke, A.; Sterk, P.J.; Bel, E.H. Non-atopic males with adult onset asthma are at risk of persistent airflow limitation. Clin. Exp. Allergy 2012, 42, 769–774. [Google Scholar] [CrossRef]
- Vazquez Garcia, G.; Blake, K. Considerations for the Child with Nonatopic Asthma. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 39–42. [Google Scholar] [CrossRef]
- Kao, Y.H.; Wu, S.C. STROBE-compliant article: Is continuity of care associated with avoidable hospitalization among older asthmatic patients? Medicine 2016, 95, e4948. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lin, H.C.; Liu, Y.S.; Lo, Y.L.; Wang, C.H.; Chang, P.J.; Lo, C.Y.; Lin, S.M. Proximity to Heavy Traffic Roads and Patient Characteristics of Late of Onset Asthma in an Urban Asthma Center. Front. Med. 2021, 8, 783720. [Google Scholar] [CrossRef] [PubMed]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H. An official American Thoracic Society/European Respiratory Society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef] [PubMed]
- Maison, N.; Omony, J.; Illi, S.; Thiele, D.; Skevaki, C.; Dittrich, A.M.; Bahmer, T.; Rabe, K.F.; Weckmann, M.; Happle, C. T2-high asthma phenotypes across life span. Eur. Respir. J. 2022, 60, 2102288. [Google Scholar] [CrossRef] [PubMed]
- Ulrik, C.S.; Lange, P. Decline of lung function in adults with bronchial asthma. Am. J. Respir. Crit. Care Med. 1994, 150, 629–634. [Google Scholar] [CrossRef]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Rahman, K.; Abejie, B.; Jain, V.V.; Vempilly, J.J. Longer duration of asthma is significantly associated with increased RV/TLC ratio. Respir. Med. 2017, 124, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway remodeling in asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Drews, A.; Pizzichini, M.; Pizzichini, E.; Pereira, M.; Pitrez, P.; Jones, M.; Sly, P.; Stein, R. Neutrophilic airway inflammation is a main feature of induced sputum in nonatopic asthmatic children. Allergy 2009, 64, 1597–1601. [Google Scholar] [CrossRef]
- Loureiro, C.; Amaral, L.; Ferreira, J.; Lima, R.; Pardal, C.; Fernandes, I.; Semedo, L.; Arrobas, A. Omalizumab for severe asthma: Beyond allergic asthma. BioMed Res. Int. 2018, 2018, 3254094. [Google Scholar] [CrossRef]
- Bumbacea, D.; Campbell, D.; Nguyen, L.; Carr, D.; Barnes, P.; Robinson, D.; Chung, K. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur. Respir. J. 2004, 24, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.F.; Phanareth, K.; Laursen, L.C.; Kok-Jensen, A.; Dirksen, A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).