Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Study, Design, and Data Collection
2.2. Inclusion and Exclusion Criteria, Study Endpoints
2.3. Measurement of Plasma Lactate
2.4. Statistical Methods
3. Results
3.1. Study Population
3.2. Association with Clinical and Laboratory Data
3.3. Prognostic Performance of Lactate Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiele, H. Editorial: Cardiogenic shock: On the search for a breakthrough in outcome? Curr. Opin. Crit. Care 2019, 25, 363–364. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, S.; Thiele, H. An overview of international cardiogenic shock guidelines and application in clinical practice. Curr. Opin. Crit. Care 2019, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Chalkias, A.; Spyropoulos, V.; Koutsovasilis, A.; Papalois, A.; Kouskouni, E.; Xanthos, T. Cardiopulmonary Arrest and Resuscitation in Severe Sepsis and Septic Shock: A Research Model. Shock 2015, 43, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Dopp-Zemel, D.; Groeneveld, A.B. High-dose norepinephrine treatment: Determinants of mortality and futility in critically ill patients. Am. J. Crit. Care 2013, 22, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Fuernau, G. Lactate and other biomarkers as treatment target in cardiogenic shock. Curr. Opin. Crit. Care 2019, 25, 403–409. [Google Scholar] [CrossRef]
- Moskowitz, A.; Omar, Y.; Chase, M.; Lokhandwala, S.; Patel, P.; Andersen, L.W.; Cocchi, M.N.; Donnino, M.W. Reasons for death in patients with sepsis and septic shock. J. Crit. Care 2017, 38, 284–288. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Burstein, B.; Vallabhajosyula, S.; Ternus, B.; Barsness, G.W.; Kashani, K.; Jentzer, J.C. The Prognostic Value of Lactate in Cardiac Intensive Care Unit Patients With Cardiac Arrest and Shock. Shock 2021, 55, 613–619. [Google Scholar] [CrossRef]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Buttner, P.; et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Poss, J.; Koster, J.; Fuernau, G.; Eitel, I.; de Waha, S.; Ouarrak, T.; Lassus, J.; Harjola, V.P.; Zeymer, U.; Thiele, H.; et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Lassus, J.; Sionis, A.; Kober, L.; Tarvasmaki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Attana, P.; Lazzeri, C.; Chiostri, M.; Picariello, C.; Gensini, G.F.; Valente, S. Lactate clearance in cardiogenic shock following ST elevation myocardial infarction: A pilot study. Acute Card. Care 2012, 14, 20–26. [Google Scholar] [CrossRef]
- Fuernau, G.; Desch, S.; de Waha-Thiele, S.; Eitel, I.; Neumann, F.J.; Hennersdorf, M.; Felix, S.B.; Fach, A.; Bohm, M.; Poss, J.; et al. Arterial Lactate in Cardiogenic Shock: Prognostic Value of Clearance Versus Single Values. JACC Cardiovasc. Interv. 2020, 13, 2208–2216. [Google Scholar] [CrossRef]
- Lindholm, M.G.; Hongisto, M.; Lassus, J.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; Salvatore, D.; Sionis, A.; et al. Serum Lactate and A Relative Change in Lactate as Predictors of Mortality in Patients With Cardiogenic Shock—Results from the Cardshock Study. Shock 2020, 53, 43–49. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Kashani, K.B.; Wiley, B.M.; Patel, P.C.; Baran, D.A.; Barsness, G.W.; Henry, T.D.; Van Diepen, S. Laboratory Markers of Acidosis and Mortality in Cardiogenic Shock: Developing a Definition of Hemometabolic Shock. Shock 2022, 57, 31–40. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Schrage, B.; Patel, P.C.; Kashani, K.B.; Barsness, G.W.; Holmes, D.R., Jr.; Blankenberg, S.; Kirchhof, P.; Westermann, D. Association Between the Acidemia, Lactic Acidosis, and Shock Severity with Outcomes in Patients with Cardiogenic Shock. J. Am. Heart Assoc. 2022, 11, e024932. [Google Scholar] [CrossRef]
- Fuller, B.M.; Dellinger, R.P. Lactate as a hemodynamic marker in the critically ill. Curr. Opin. Crit. Care 2012, 18, 267–272. [Google Scholar] [CrossRef]
- Kliegel, A.; Losert, H.; Sterz, F.; Holzer, M.; Zeiner, A.; Havel, C.; Laggner, A.N. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine 2004, 83, 274–279. [Google Scholar] [CrossRef]
- Issa, M.S.; Grossestreuer, A.V.; Patel, H.; Ntshinga, L.; Coker, A.; Yankama, T.; Donnino, M.W.; Berg, K.M. Lactate and hypotension as predictors of mortality after in-hospital cardiac arrest. Resuscitation 2021, 158, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Miller, J.; Goyal, N.; Loomba, M.; Sankey, S.S.; Dolcourt, B.; Sherwin, R.; Otero, R.; Wira, C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation 2007, 75, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.N.; Salciccioli, J.; Yankama, T.; Chase, M.; Patel, P.V.; Liu, X.; Mader, T.J.; Donnino, M.W. Predicting Outcome After Out-of-Hospital Cardiac Arrest: Lactate, Need for Vasopressors, and Cytochrome c. J. Intensive Care Med. 2020, 35, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Lonsain, W.S.; De Lausnay, L.; Wauters, L.; Desruelles, D.; Dewolf, P. The prognostic value of early lactate clearance for survival after out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2021, 46, 56–62. [Google Scholar] [CrossRef]
- Torre, T.; Toto, F.; Klersy, C.; Theologou, T.; Casso, G.; Gallo, M.; Surace, G.G.; Franciosi, G.; Demertzis, S.; Ferrari, E. Early predictors of mortality in refractory cardiogenic shock following acute coronary syndrome treated with extracorporeal membrane oxygenator. J. Artif. Organs 2021, 24, 327–335. [Google Scholar] [CrossRef]
- Lauridsen, M.D.; Josiassen, J.; Schmidt, M.; Butt, J.H.; Ostergaard, L.; Schou, M.; Kjaergaard, J.; Moller, J.E.; Hassager, C.; Torp-Pedersen, C.; et al. Prognosis of myocardial infarction-related cardiogenic shock according to preadmission out-of-hospital cardiac arrest. Resuscitation 2021, 162, 135–142. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S.; Henry, T.D. Understanding How Cardiac Arrest Complicates the Analysis of Clinical Trials of Cardiogenic Shock. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006692. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Henry, T.D.; Barsness, G.W.; Menon, V.; Baran, D.A.; Van Diepen, S. Influence of cardiac arrest and SCAI shock stage on cardiac intensive care unit mortality. Catheter. Cardiovasc. Interv. 2020, 96, 1350–1359. [Google Scholar] [CrossRef]
- Nichol, A.; Bailey, M.; Egi, M.; Pettila, V.; French, C.; Stachowski, E.; Reade, M.C.; Cooper, D.J.; Bellomo, R. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit. Care 2011, 15, R242. [Google Scholar] [CrossRef]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: A document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 183–197. [Google Scholar] [CrossRef]
- Abdi, H. The greenhouse-geisser correction. Encycl. Res. Des. 2010, 1, 544–548. [Google Scholar]
- Mizock, B.A.; Falk, J.L. Lactic acidosis in critical illness. Crit. Care Med. 1992, 20, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Revelly, J.P.; Tappy, L.; Martinez, A.; Bollmann, M.; Cayeux, M.C.; Berger, M.M.; Chiolero, R.L. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit. Care Med. 2005, 33, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.; Wulf, M.E. Lactic acidosis in sepsis: A commentary. Intensive Care Med. 1996, 22, 6–16. [Google Scholar] [CrossRef]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef]
- Vincent, J.L.; De Backer, D. Circulatory shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Jankowski, M. B-type natriuretic peptide for diagnosis and therapy. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 77–83. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef]
| All Patients (n = 263) | OHCA (n = 101) | Non-OHCA (n = 162) | ||||
|---|---|---|---|---|---|---|
| Age, median; (IQR) | 73 | (63–81) | 66 | (57–78) | 77 | (67–82) |
| Male sex, n (%) | 158 | (60.0) | 74 | (73.3) | 84 | (51.9) |
| Body mass index, kg/m2 (median, (IQR)) | 26.20 | (24.20–30.00) | 26.25 | (24.23–29.88) | 26.20 | (24.20–30.05) |
| Physiological parameters, (median, (IQR)) | ||||||
| Body temperature (°C) | 36.0 | (34.9–36.5) | 35.2 | (34.0–36.2) | 36.2 | (35.6–36.8) |
| Heart rate (bpm) | 88 | (71–108) | 84 | (71–106) | 90 | (71–110) |
| Systolic blood pressure (mmHg) | 109 | (92–129) | 114 | (98–135) | 105 | (86–127) |
| Respiratory rate (breaths/min) | 20 | (17–24) | 19 | (16–22) | 20 | (17–25) |
| Cardiovascular risk factors, n (%) | ||||||
| Arterial hypertension | 193 | (73.4) | 64 | (63.4) | 129 | (79.6) |
| Diabetes mellitus | 103 | (39.2) | 28 | (28.0) | 75 | (46.3) |
| Hyperlipidemia | 136 | (51.7) | 40 | (39.6) | 96 | (59.3) |
| Smoking | 95 | (36.1) | 30 | (29.7) | 65 | (40.1) |
| Prior medical history, n (%) | ||||||
| Coronary artery disease | 98 | (37.3) | 31 | (30.7) | 67 | (41.4) |
| Congestive heart failure | 93 | (35.4) | 18 | (17.8) | 75 | (46.3) |
| Atrial fibrillation | 85 | (32.3) | 20 | (19.8) | 65 | (40.1) |
| Chronic kidney disease | 89 | (33.9) | 16 | (15.8) | 73 | (45.1) |
| Stroke | 36 | (13.7) | 5 | (5.0) | 31 | (19.1) |
| COPD | 51 | (19.4) | 18 | (17.8) | 33 | (20.4) |
| Liver cirrhosis | 9 | (3.4) | 2 | (2.0) | 7 | (4.3) |
| Medication on admission, n (%) | ||||||
| ACE-inhibitor | 92 | (35.0) | 29 | (33.3) | 63 | (38.9) |
| ARB | 46 | (17.5) | 12 | (13.6) | 34 | (21.0) |
| Beta-blocker | 132 | (50.2) | 33 | (37.9) | 99 | (61.1) |
| ARNI | 8 | (3.0) | 2 | (2.3) | 6 | (3.7) |
| Aldosterone antagonist | 40 | (15.2) | 13 | (14.9) | 27 | (16.7) |
| Diuretics | 114 | (43.3) | 26 | (29.5) | 88 | (54.3) |
| ASA | 76 | (28.9) | 18 | (17.8) | 58 | (35.8) |
| P2Y12-inhibitor | 23 | (8.8) | 7 | (6.9) | 16 | (9.9) |
| Statin | 117 | (44.5) | 32 | (31.7) | 85 | (52.4) |
| All Patients (n = 263) | OHCA (n = 101) | Non-OHCA (n = 162) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Cause of CS, n (%) | |||||||
| Acute myocardial infarction | 126 | (47.9) | 58 | (57.4) | 69 | (42.6) | |
| Arrhythmic | 31 | (11.8) | 12 | (11.9) | 19 | (11.7) | |
| ADHF | 65 | (24.7) | 16 | (15.8) | 49 | (30.2) | |
| Pulmonary embolism | 15 | (5.7) | 9 | (8.9) | 6 | (3.7) | 0.003 |
| Valvular defect | 12 | (4.6) | 1 | (1.0) | 11 | (6.8) | |
| Cardiomyopathy | 7 | (2.7) | 4 | (4.0) | 3 | (1.9) | |
| Aortic dissection | 6 | (2.3) | 1 | (1.0) | 5 | (3.1) | |
| SCAI-Classification of CS, n (%) | |||||||
| Stage A | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0.001 |
| Stage B | 6 | (0.0) | 0 | (0.0) | 6 | (3.7) | |
| Stage C | 95 | (36.1) | 0 | (0.0) | 95 | (58.6) | |
| Stage D | 20 | (7.6) | 0 | (0.0) | 20 | (12.3) | |
| Stage E | 142 | (54.0) | 101 | (100.0) | 41 | (25.3) | |
| Transthoracic echocardiography | |||||||
| LVEF > 55%, (n, %) | 27 | (10.2) | 8 | (7.9) | 19 | (11.7) | |
| LVEF 54–41%, (n, %) | 31 | (11.8) | 11 | (10.9) | 20 | (12.3) | |
| LVEF 40–30%, (n, %) | 61 | (23.2) | 25 | (24.8) | 36 | (22.2) | 0.483 |
| LVEF < 30%, (n, %) | 126 | (47.9) | 47 | (46.5) | 79 | (48.8) | |
| LVEF not documented, (n, %) | 18 | (6.8) | 10 | (9.9) | 8 | (4.9) | |
| VCI, cm (median, (IQR)) | 1.8 | (1.5–2.2) | 1.8 | (1.5–2.2) | 1.9 | (1.6–2.2) | 0.099 |
| TAPSE, mm (median, (IQR)) | 15 | (11–18) | 17 | (14–21) | 14 | (11–18) | 0.015 |
| Cardiopulmonary resuscitation | |||||||
| OHCA, n (%) | 101 | (38.4) | 101 | (100.0) | 0 | (0.0) | 0.001 |
| IHCA, n (%) | 41 | (15.6) | 0 | (0.0) | 41 | (25.3) | |
| No CPR, n (%) | 121 | (46.0) | 0 | (0.0) | 121 | (74.7) | |
| Shockable rhythm, n (%) | 72 | (27.6) | 61 | (61.0) | 11 | (6.8) | 0.001 |
| Non-shockable rhythm, n (%) | 70 | (26.6) | 40 | (39.6) | 30 | (18.5) | |
| ROSC, min (median, IQR) | 15 | (10–27) | 15 | (10–29) | 10 | (5–23) | 0.028 |
| Respiratory status | |||||||
| Mechanical ventilation, n (%) | 151 | (57.4) | 96 | (95.1) | 55 | (34.8) | 0.001 |
| Duration of mechanical ventilation, days (median, (IQR)) | 2 | (1–6) | 5 | (2–9) | 1 | (0–2) | 0.001 |
| PaO2/FiO2 ratio, (median, (IQR)) | 222 | (133–354) | 192 | (120–331) | 252 | (142–367) | 0.166 |
| PaO2, mmHg (median, (IQR)) | 103 | (77–165) | 112 | (79–201) | 98 | (76–149) | 0.047 |
| Multiple organ support during ICU | |||||||
| Dosis norepinephrine on admission, µg/kg/min (median, (IQR)) | 0.1 | (0.0–0.3) | 0.2 | (0.1–0.4) | 0.1 | (0.0–0.2) | 0.001 |
| Levosimendan, n (%) | 68 | (25.9) | 22 | (21.8) | 46 | (28.4) | 0.234 |
| Mechanical circulatory assist device, n (%) | 25 | (9.5) | 13 | (12.9) | 12 | (7.4) | 0.142 |
| CRRT, n (%) | 79 | (30.0) | 29 | (28.7) | 50 | (30.9) | 0.711 |
| Baseline laboratory values, (median, (IQR)) | |||||||
| pH | 7.29 | (7.19–7.37) | 7.25 | (7.15–7.34) | 7.31 | (7.23–7.38) | 0.001 |
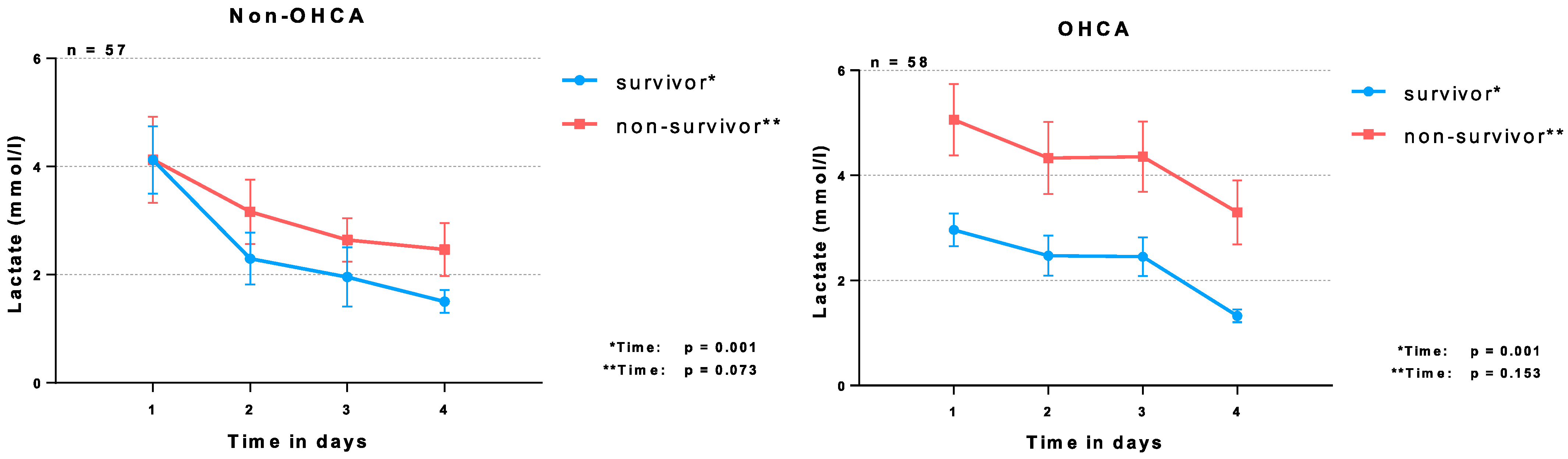
| Lactate (mmol/L) | 3.3 | (1.7–7.2) | 3.8 | (2.2–8.8) | 2.8 | (1.6–6.1) | 0.021 |
| Serum sodium (mmol/L) | 138 | (136–141) | 139 | (136–140) | 137 | (135–141) | 0.132 |
| Serum potassium (mmol/L) | 4.3 | (3.8–4.9) | 4.2 | (3.5–4.6) | 4.4 | (4.0–5.0) | 0.004 |
| Serum creatinine (mg/dL) | 1.48 | (1.12–2.14) | 1.41 | (1.18–1.76) | 1.53 | (1.10–2.85) | 0.037 |
| Hemoglobin (g/dL) | 12.4 | (10.3–13.9) | 13.2 | (11.5–14.4) | 11.9 | (9.7–13.7) | 0.001 |
| WBC (106/mL) | 14.71 | (10.49–19.05) | 16.39 | (12.31–21.81) | 13.31 | (9.93–17.85) | 0.001 |
| Platelets (106/mL) | 222 | (171–275) | 225 | (175–276) | 215 | (165–273) | 0.726 |
| INR | 1.17 | (1.08–1.39) | 1.17 | (1.08–1,35) | 1.18 | (1.09–1.41) | 0.364 |
| D-dimer (mg/L) | 9.78 | (2.43–32.00) | 24.18 | (14.28–32.00) | 2.86 | (1.23–6.87) | 0.001 |
| AST (U/L) | 130 | (43–324) | 167 | (110–416) | 94 | (32–265) | 0.005 |
| ALT (U/L) | 77 | (32–189) | 115 | (71–202) | 51 | (27–182) | 0.001 |
| Bilirubin (mg/dL) | 0.63 | (0.43–1.00) | 0.61 | (0.41–0.79) | 0.66 | (0.43–1.19) | 0.061 |
| Troponin I (µg/L) | 0.763 | (0.164–6.154) | 0.731 | (0.201–5.047) | 0.780 | (0.143–8.849) | 0.800 |
| NT-pro BNP (pg/mL) | 4387 | (729–13,595) | 1047 | (339–4462) | 7944 | (2459–15,352) | 0.001 |
| Procalcitonin (ng/mL) | 0.28 | (0.11–0.96) | 0.23 | (0.06–1.22) | 0.31 | (0.12–0.98) | 0.594 |
| CRP (mg/L) | 13 | (4–43) | 4 | (4–19) | 25 | (5–69) | 0.001 |
| Primary endpoint | |||||||
| All-cause mortality at 30 days, n (%) | 144 | (54.8) | 64 | (63.4) | 80 | (49.4) | 0.027 |
| Follow up data, n (%) | |||||||
| ICU time, days (median, (IQR)) | 4 | (2–8) | 6 | (3–10) | 3 | (2–6) | 0.001 |
| Death ICU, n (%) | 143 | (54.4) | 64 | (63.4) | 79 | (48.8) | 0.021 |
| Non-OHCA | OHCA | |||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Age | 0.038 | 0.630 | −0.183 | 0.067 |
| BMI | 0.116 | 0.147 | 0.068 | 0.511 |
| WBC (106/mL) | 0.052 | 0.528 | 0.087 | 0.404 |
| Platelet count (106/mL) | −0.174 | 0.034 | −0.289 | 0.005 |
| Albumin (g/L) | −0.177 | 0.040 | −0.465 | 0.001 |
| Bilirubin (mg/dL) | 0.295 | 0.003 | 0.340 | 0.012 |
| CRP (mg/L) | 0.113 | 0.182 | 0.203 | 0.050 |
| Procalcitonin (ng/mL) | 0.226 | 0.091 | 0.551 | 0.018 |
| cTNI (µg/L) | −0.012 | 0.898 | 0.264 | 0.010 |
| NT-pro BNP (pg/mL) | 0.145 | 0.228 | 0.291 | 0.112 |
| LVEF | 0.168 | 0.033 | 0.224 | 0.025 |
| PaO2/FiO2 ratio | −0.024 | 0.779 | −0.269 | 0.011 |
| Mechanical ventilation days | 0.039 | 0.623 | −0.407 | 0.001 |
| Creatinine (mg/dL) | 0.294 | 0.001 | 0.240 | 0.019 |
| SOFA score | 0.296 | 0.001 | 0.283 | 0.004 |
| APACHE II score | 0.367 | 0.001 | 0.101 | 0.313 |
| MAP (mmHg) | −0.104 | 0.194 | −0.197 | 0.057 |
| Catecholamines | 0.258 | 0.001 | 0.253 | 0.012 |
| Intensive care days | −0.261 | 0.001 | −0.441 | 0.001 |
| Non-OHCA | OHCA | |
|---|---|---|
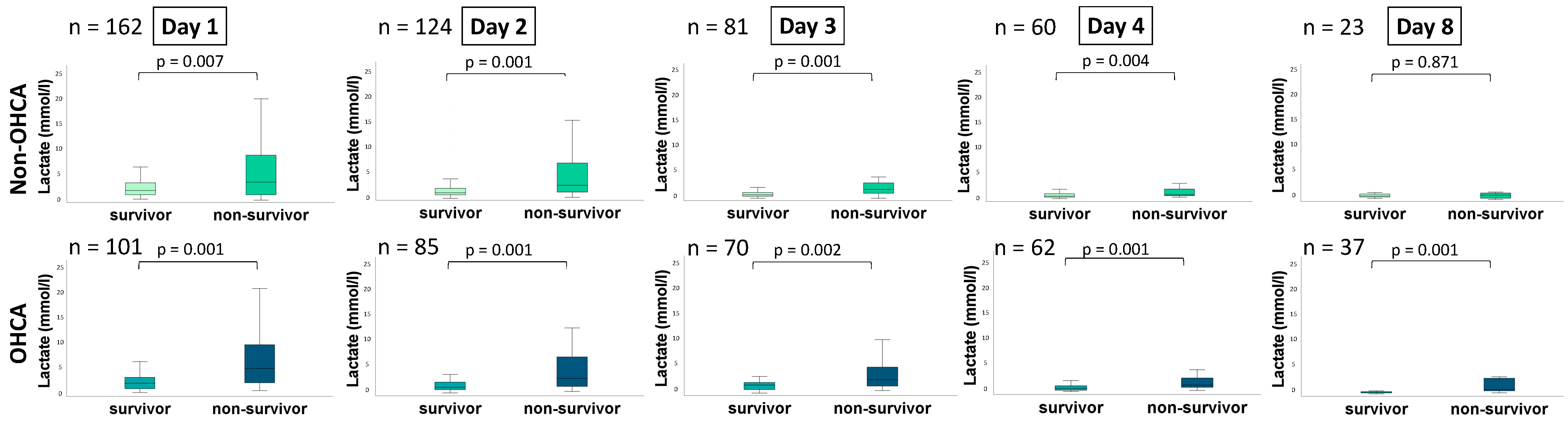
| Day 1 | 0.622 (0.535–0.710); p = 0.007 | 0.753 (0.658–0.848); p = 0.001 |
| Day 2 | 0.758 (0.672–0.843), p = 0.001 | 0.731 (0.624–0.839); p = 0.001 |
| Day 3 | 0.784 (0.679–0.889); p = 0.001 | 0.720 (0.601–0.838); p = 0.002 |
| Day 4 | 0.722 (0.592–0.852); p = 0.004 | 0.761 (0.644–0.877); p = 0.001 |
| Day 8 | 0.479 (0.204–0.754); p = 0.872 | 0.863 (0.739–0.987); p = 0.001 |
| Variables | Non-OHCA | OHCA | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.020 | 0.997–1.044 | 0.087 | 1.022 | 1.000–1.045 | 0.052 |
| Sex | 0.930 | 0.553–1.565 | 0.786 | 1.068 | 0.585–1.947 | 0.831 |
| WBC | 1.078 | 1.032–1.125 | 0.001 | 1.005 | 0.959–1.053 | 0.836 |
| Platelets | 1.000 | 0.997–1.003 | 0.873 | 0.999 | 0.995–1.002 | 0.406 |
| Systolic BP | 0.993 | 0.984–1.002 | 0.121 | 0.991 | 0.983–1.000 | 0.047 |
| Respiratory rate | 1.024 | 0.984–1.065 | 0.246 | 1.001 | 0.955–1.050 | 0.968 |
| Creatinine | 1.028 | 0.904–1.170 | 0.670 | 1.100 | 0.880–1.377 | 0.403 |
| Catecholamines | 1.390 | 1.094–1.768 | 0.007 | 1.006 | 0.742–1.363 | 0.972 |
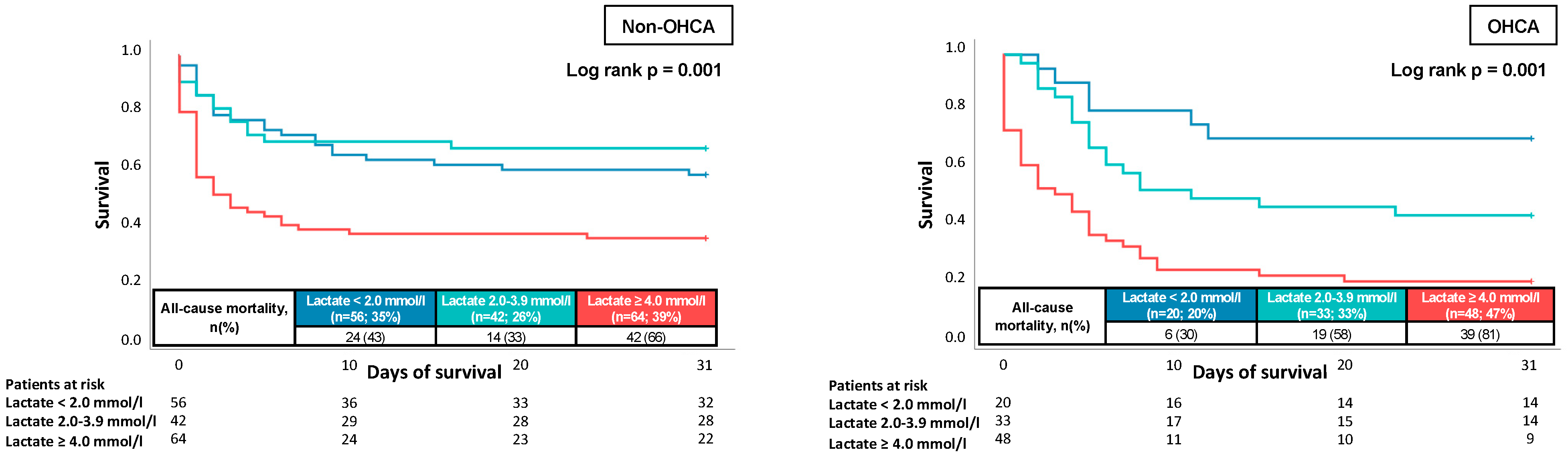
| Lactate | 1.070 | 1.009–1.134 | 0.024 | 1.151 | 1.071–1.238 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusnak, J.; Schupp, T.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Forner, J.; Bertsch, T.; Kittel, M.; Mashayekhi, K.; Tajti, P.; et al. Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock. J. Clin. Med. 2022, 11, 7295. https://doi.org/10.3390/jcm11247295
Rusnak J, Schupp T, Weidner K, Ruka M, Egner-Walter S, Forner J, Bertsch T, Kittel M, Mashayekhi K, Tajti P, et al. Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock. Journal of Clinical Medicine. 2022; 11(24):7295. https://doi.org/10.3390/jcm11247295
Chicago/Turabian StyleRusnak, Jonas, Tobias Schupp, Kathrin Weidner, Marinela Ruka, Sascha Egner-Walter, Jan Forner, Thomas Bertsch, Maximilian Kittel, Kambis Mashayekhi, Péter Tajti, and et al. 2022. "Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock" Journal of Clinical Medicine 11, no. 24: 7295. https://doi.org/10.3390/jcm11247295
APA StyleRusnak, J., Schupp, T., Weidner, K., Ruka, M., Egner-Walter, S., Forner, J., Bertsch, T., Kittel, M., Mashayekhi, K., Tajti, P., Ayoub, M., Behnes, M., & Akin, I. (2022). Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock. Journal of Clinical Medicine, 11(24), 7295. https://doi.org/10.3390/jcm11247295







