Conversion to mTOR-Inhibitors Plus IV Immunoglobulins in Kidney-Transplant Recipients with BKV Infection: A Retrospective Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. BKV Quantification
2.3. Endpoints
2.4. Immunosuppression
2.5. Statistical Analyses
3. Results
3.1. Cohort Characteristics
3.1.1. Clinical Characteristics
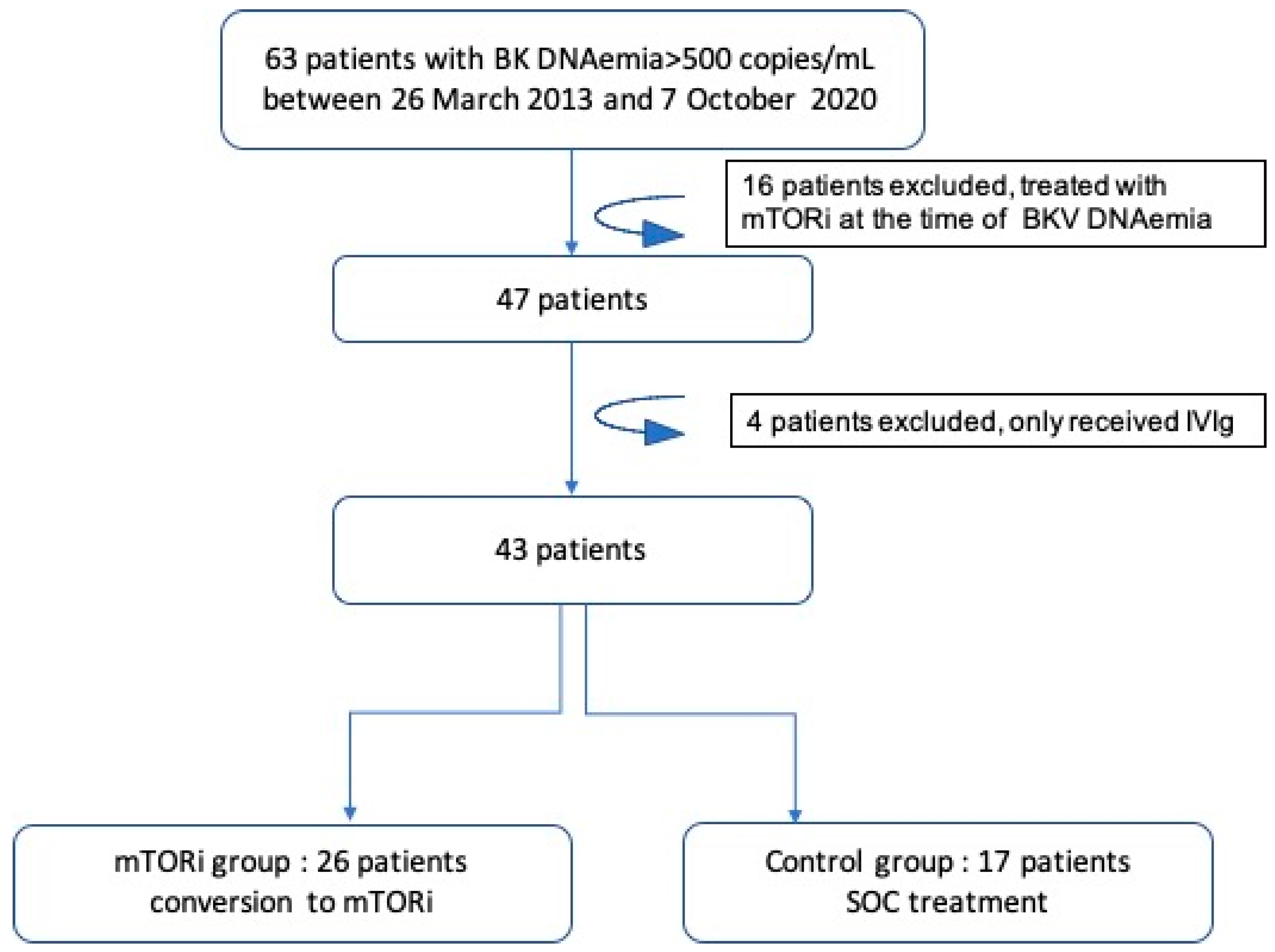
- Between 26 March 2013 and 7 October 2020, 67 patients had a positive BKV blood DNAemia of >500 copies/mL (22%). Forty-seven patients did not have an mTORi-based regimen at the time of the first incidence of DNAemia. Among these, 26 patients were converted to mTORi with or without simultaneous infusion of IVIgs (mTORi±IVIg group) and 17 underwent reduced immunosuppression (control group, i.e., standard of care). Eighteen patients (69%) received IVIgs as well as mTOR. The t//Total IVIg mean dose was 94.7 ± 60 g (1.3 g/kg).
- Ten patients did not meet the inclusion criteria (see lowchart in Figure 1). Baseline characteristics of the included population are shown in Table 1. Clinical characteristics, such as age at time of DNAemia, gender, body-mass index (BMI), initial nephropathy, diabetes mellitus pre-KT, and vascular impairment, were similar between the two groups. The mTORi±IVIg group was more likely to have received a graft from a living donor, a younger donor, an ABO-incompatible donor and to receive Rituximab after KT as compared to the control group.
- Median follow-up time of the study population from medical intervention until the last follow-up was 45 (25.7–70.5) months: with a shorter time for the mTORi±IVIg group compared to the control group (respectively, 32.3 (23.8–47.7)/] vs. 77.3 (64.6–91) months, p < 0.001).
3.1.2. BKV at the Time of Medical Intervention
- BKV DNAemia at the time of medical intervention did not significantly differ between the mTORi±IVIg group and the control group: i.e., respectively, 3.8 ± 0.9 log10 copies/mL vs. 4.2 ± 0.6 log10 copies/mL, p = 0.15. Biopsy-proven PvAN (SV40+) at the time of medical intervention did not significantly differ between the two groups: ten patients (38%) in the mTORi±IVIg group versus one patient (6%) in the control group (p = 0.28).
3.2. Primary Endpoint and BKV DNAemia Outcomes
- The median times between KT and BKV DNAemia when medical intervention was conducted were similar: i.e., 3.6 (2.8–7.1) months for the mTORi±IVIg group, and 3.6 (3.0–4.6) months for the control group (p = 0.84).
- Regarding the primary endpoint, the prevalence of BKV DNAemia clearance during the first year post-medical intervention was significantly higher in the control group compared to the mTORi±IVIg group (88% vs. 58%; p = 0.03).
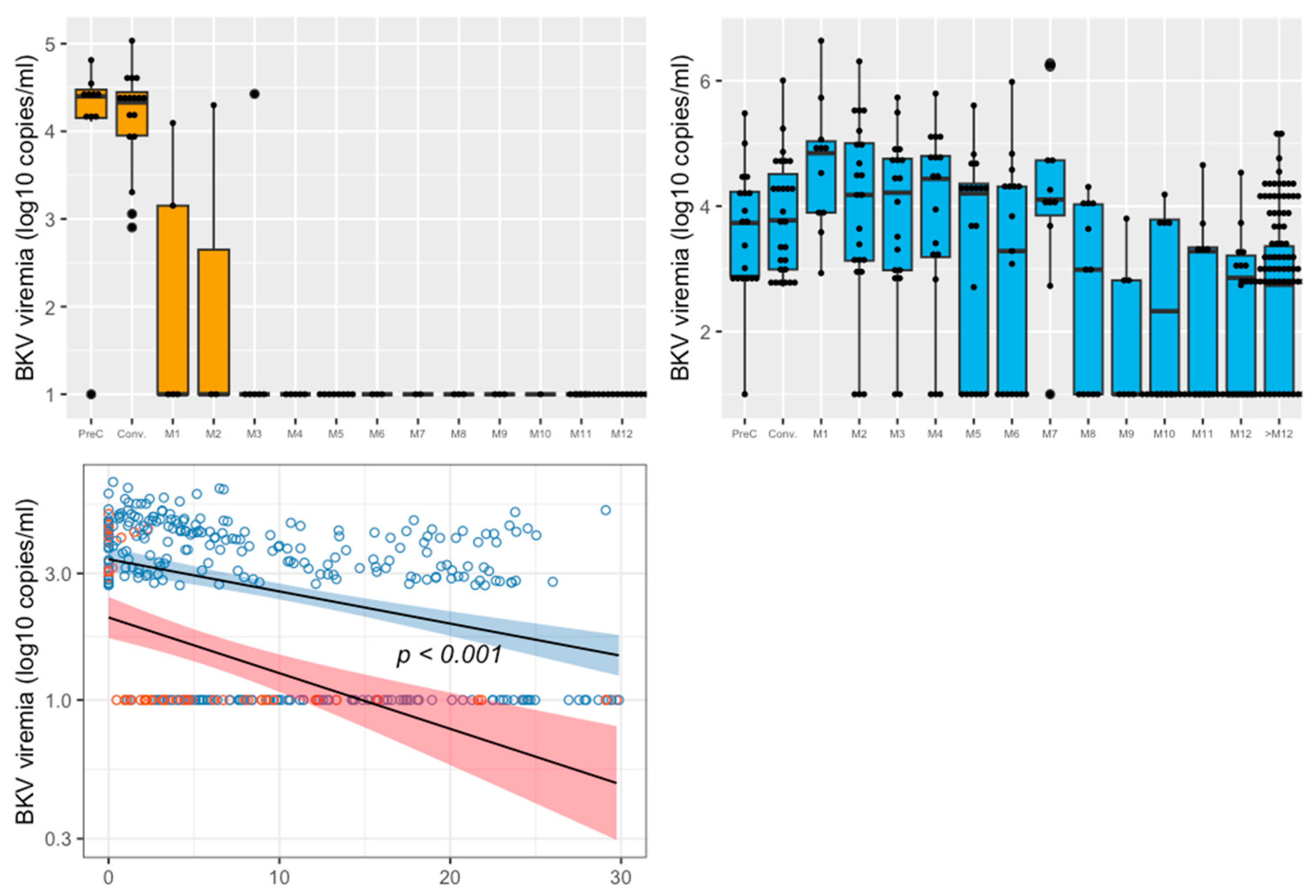
- Figure 2 represents the kinetics of BKV blood DNAemia over time for both groups. Using a linear regression model, we showed that the decreasing slopes of BKV DNAemia over time differed significantly between the mTORi±IVIg and control groups, in favor of the control group (p < 0.001).
- At month 12 post-medical intervention, the viral load was 2.3 ± 1.3 log10 copies/mL in the mTORi±IVIg group, whereas no patient had positive BKV DNAemia in the control group (p = 0.10). We then assessed, for each month, the BKV DNAemia level in blood after the medical intervention (Figure 2). In the control group, BKV decreased rapidly at month 3 to then remain negative for beyond one year. Conversely, in the mTORi±IVIg group, BKV DNAemia decreased more slowly, and many more patients still hadll positive viral loads after one year. Mean BKV DNAemia in the mTORi±IVIg group was 3.8 (1.4) log10/mL at month 3, at 2.9 (1.8) log10/mL at month 6, at 2.3 (1.3) log10/mL at month 12, and 2.3 (1.4) log10/mL beyond the first year after medical intervention.
- During the follow-up, three patients (11%) developed biopsy-proven PvAN: all were in the mTORi±IVIg group.
3.3. Kidney Outcomes
3.3.1. Graft Survival
3.3.2. Rejection Rate after the Medical Intervention
3.3.3. Kidney Function
- At the time of medical intervention, mean eGFR was 56 ± 19 mL/min/1.73 m2. There was no statistical difference in eGFR between the groups: 58.5 ± 19 mL/min/1.73 m2 in the mTORi±IVIg group and 52.8 ± 18.2 mL/min/1.73 m2 in the control group (p = 0.325). Similarly, the median proteinuria level at the time of medical intervention was 0.3 [0.1–0.3] g/L. Proteinuria also did not statistically differ between the two groups: 0.3 [0.2–0.3] g/L versus 0.2 [0–0.3] g/L in the mTORi±IVIg group and control groups, respectively (p = 0.09).
- Kidney-graft function at 12 months post-medical intervention did not significantly differ between the mTORi±IVIg and control groups, respectively: 54.3 ± 19.4 mL/min/1.73 m2 and 51.5 ± 24.4 mL/min/1.73 m2 (p = 0.468). Proteinuria at 12 months post-medical intervention was significantly higher in the mTORi±IVIg group compared to the control group: 0.3 [0.2–0.8] g/L versus 0.1 [0–0.2] g/L, respectively, p = 0.004.
3.4. Immunosuppression Management and Tolerability
3.5. Factors Associated with BKV DNAemia Clearance
- We also assessed factors associated significantly with our primary endpoint: i.e., BKV DNAemia clearance at 1 year after medical intervention (Table 3).
- In univariate analysis, receiving a kidney from a female donor and reduced immunosuppression versus mTORi conversion were significantly associated with BKV DNAemia clearance. Time of occurrence between transplantation and medical intervention did not significantly differ between the BKV DNAemia clearance groups: 3.6 [3.0–4.6] months versus 5.4 [2.9–12.7] months for persistent BKV DNAemia patients (p = 0.14), nor did lymphocyte level at the time of medical intervention. The use of IVig was not associated to BKV DNAemia clearance in univariate analysis neither in a model when associated with mTOR conversion (OR = 0.33 [0.04–1.9], p = 0.24).
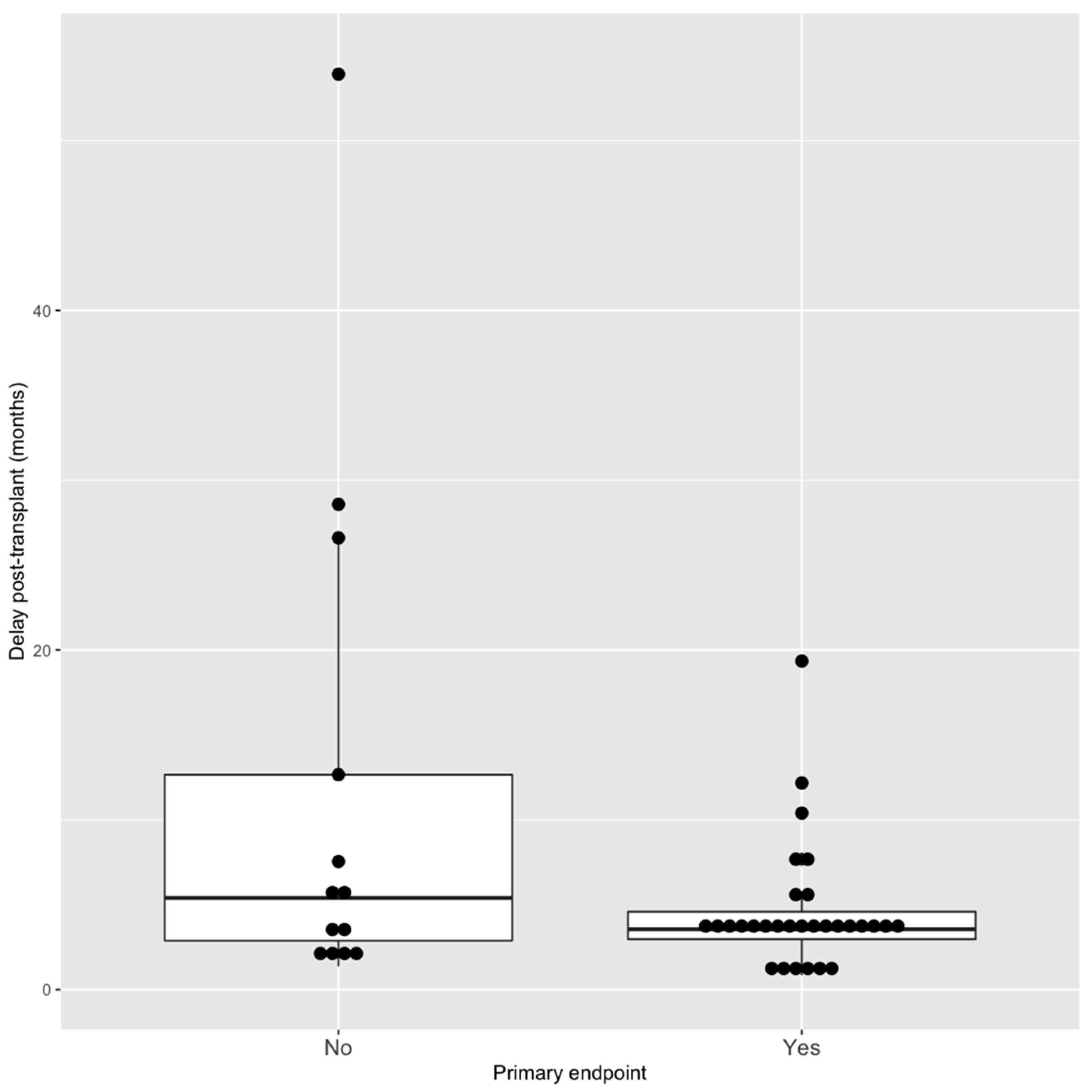
- In the multivariate model, we included all significant factors associated with BKV clearance and we included the delay of BKV occurrence post transplantation because the association was really close to significance and a shorter delay of initial viremia appeared to be significantly associated with BKV endpoint. Three factors remained significantly associated with less BKV DNAemia clearance at 1 year in this model: mTORi conversion (vs. reducing immunosuppression), having a male donor (as defined in Section 2) and a shorter delay of BKV DNAemia occurrence post transplantation (Figure 4): OR = 0.11 [0.06–0.9] p = 0.045 and 0.10 [0.01–0.59] p = 0.018 and 0.88 [0.76–0.96] p = 0.019, respectively.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halloran, P.F. Immunosuppressive Drugs for Kidney Transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.D.; Field, A.M.; Coleman, D.V. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1970, 297, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.F.; Borden, E.C.; McBain, J.A.; Padgett, B.L.; Walker, D.L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann. Intern. Med. 1980, 92, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Steiger, J. Polyomavirus BK. Lancet Infect. Dis. 2003, 3, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y. Bk virus-associated nephropathy after renal transplantation. Pathogens 2021, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H. Polyomavirus BK Nephropathy: A (Re-) emerging Complication in Renal Transplantation. Am. J. Transplant. 2002, 2, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Agha, I.; Bohl, D.L.; Schnitzler, M.A.; Hardinger, K.L.; Lockwood, M.; Torrence, S.; Schuessler, R.; Roby, T.; Gaudreault-Keener, M.; et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 2005, 5, 582–594. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Randhawa, P.S. BK polyomavirus in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef]
- Lee, H.M.; Jang, I.A.; Lee, D.; Kang, E.J.; Choi, B.S.; Park, C.W.; Choi, Y.J.; Yang, C.W.; Kim, Y.S.; Chung, B.H. Risk factors in the progression of BK virus-associated nephropathy in renal transplant recipients. Korean J. Intern. Med. 2015, 30, 865–872. [Google Scholar] [CrossRef]
- Johnston, O.; Jaswal, D.; Gill, J.S.; Doucette, S.; Fergusson, D.A.; Knoll, G.A. Treatment of Polyomavirus Infection in Kidney Transplant Recipients: A Systematic Review. Transplantation 2010, 89, 1057–1070. [Google Scholar] [CrossRef]
- Shen, C.L.; Wu, B.S.; Lien, T.J.; Yang, A.H.; Yang, C.Y. Bk polyomavirus nephropathy in kidney transplantation: Balancing rejection and infection. Viruses 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, S.; Astor, B.C.; Kaufman, D.; Muth, B.; Garg, N.; Djamali, A.; Mandelbrot, D.A. Which is more nephrotoxic for kidney transplants: BK nephropathy or rejection? Clin. Transplant. 2018, 32, e132163. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Yakhontova, K.; Lu, M. BK Polyomavirus Replication in Renal Tubular Epithelial Cells Is Inhibited by Sirolimus, but Activated by Tacrolimus Through a Pathway Involving FKBP-12. Am. J. Transplant. 2016, 16, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Jouve, T.; Rostaing, L.; Malvezzi, P. Place of mTOR inhibitors in management of BKV infection after kidney transplantation. J. Nephropathol. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, D.; Chandran, S.; Webber, A.; Hirose, R.; Vincenti, F. Mycophenolate Mofetil Withdrawal with Conversion to Everolimus to Treat BK Virus Infection in Kidney Transplant Recipients. Transplant. Proc. 2017, 49, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.S.; Schonder, K.; Shapiro, R.; Farasati, N.; Huang, Y. Polyomavirus bk neutralizing activity in human immunoglobulin preparations. Transplantation 2010, 89, 1462–1465. [Google Scholar] [CrossRef]
- Velay, A.; Solis, M.; Benotmane, I.; Gantner, P.; Soulier, E.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Intravenous immunoglobulin administration significantly increases BKPyV genotype-specific neutralizing antibody titers in kidney transplant recipients. Antimicrob. Agents Chemother. 2019, 63, e00393-19. [Google Scholar] [CrossRef]
- Sener, A.; House, A.A.; Jevnikar, A.M.; Boudville, N.; McAlister, V.C.; Muirhead, N.; Rehman, F.; Luke, P.P.W. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: One-year follow-up of renal allograft recipients. Transplantation 2006, 81, 117–120. [Google Scholar] [CrossRef]
- Anyaegbu, E.I.; Almond, P.S.; Milligan, T.; Allen, W.R.; Gharaybeh, S.; Al-Akash, S.I. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr. Transplant. 2012, 16, 19–24. [Google Scholar] [CrossRef]
- Vu, D.; Shah, T.; Ansari, J.; Naraghi, R.; Min, D. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant. Proc. 2015, 47, 394–398. [Google Scholar] [CrossRef]
- Hwang, S.D.; Lee, J.H.; Lee, S.W.; Kim, J.K.; Kim, M.J.; Song, J.H. High-Dose Intravenous Immunoglobulin Treatment of Polyomavirus Nephropathy Developing After T Cell–Mediated Rejection Treatment: A Case Report. Transplant. Proc. 2018, 50, 2575–2578. [Google Scholar] [CrossRef]
- Matsumura, S.; Kato, T.; Taniguchi, A.; Kawamura, M.; Nakazawa, S.; Namba-Hamano, T.; Abe, T.; Nonomura, N.; Imamura, R. Clinical efficacy of intravenous immunoglobulin for BK polyomavirus-associated nephropathy after living kidney transplantation. Ther. Clin. Risk Manag. 2020, 16, 947–952. [Google Scholar] [CrossRef]
- Moon, J.; Chang, Y.; Shah, T.; Min, D.I. Effects of intravenous immunoglobulin therapy and Fc gamma receptor polymorphisms on BK virus nephropathy in kidney transplant recipients. Transpl. Infect. Dis. 2020, 22, e13300. [Google Scholar] [CrossRef]
- Benotmane, I.; Solis, M.; Velay, A.; Cognard, N.; Olagne, J.; Gautier Vargas, G.; Perrin, P.; Marx, D.; Soulier, E.; Gallais, F.; et al. Intravenous immunoglobulin as a preventive strategy against BK virus viremia and BKV-associated nephropathy in kidney transplant recipients—Results from a proof-of-concept study. Am. J. Transplant. 2021, 21, 329–337. [Google Scholar] [CrossRef]
- Bischof, N.; Hirsch, H.H.; Wehmeier, C.; Amico, P.; Dickenmann, M.; Menter, T.; Helmut, H.; Schaub, S.; Hirt-minkowski, P. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: Long-term outcomes. Nephrol. Dial. Transplant. 2019, 34, 1240–1250. [Google Scholar] [CrossRef]
- Comoli, P.; Binggeli, S.; Ginevri, F.; Hirsch, H.H. Polyomavirus-associated nephropathy: Update on BK virus-specific immunity. Transpl. Infect. Dis. 2006, 8, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ambalathingal, G.R.; Francis, R.S.; Smyth, M.J.; Smith, C.; Khanna, R. BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies. Clin. Microbiol. Rev. 2017, 30, 503–528. [Google Scholar] [CrossRef]
- Egli, A.; Ko, S. Inhibition of Polyomavirus BK-Specific T-Cell Responses by Immunosuppressive Drugs. Transplantation 2009, 88, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Weist, B.J.; Wehler, P.; El Ahmad, L.; Schmueck-Henneresse, M.; Millward, J.M.; Nienen, M.; Neumann, A.U.; Reinke, P.; Babel, N. A revised strategy for monitoring BKV-specific cellular immunity in kidney transplant patients. Kidney Int. 2015, 88, 1293–1303. [Google Scholar] [CrossRef]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. mTOR regulates memory CD8 T cell differentiation. Nature 2010, 460, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Berger, S.P.; Witzke, O.; Tedesco, H.; Mulgaonkar, S.; Qazi, Y.; Chadban, S.; Oppenheimer, F.; Sommerer, C.; Oberbauer, R.; et al. Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation. J. Am. Soc. Nephrol. 2018, 29, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Dharnidharka, V.R.; Cherikh, W.S.; Abbott, K.C. An OPTN Analysis of National Registry Data on Treatment of BK Virus Allograft Nephropathy in the United States. Transplantation 2009, 87, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Mallat, S.G.; Tanios, B.Y.; Itani, H.S.; Lotfi, T.; McMullan, C.; Gabardi, S.; Akl, E.A.; Azzi, J.R. CMV and BKPyV infections in renal transplant recipients receiving an mtor inhibitor–based regimen versus a cni-based regimen: A systematic review and meta-analysis of randomized, controlled trials. Clin. J. Am. Soc. Nephrol. 2017, 12, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fructuoso, A.I.; Calvo, N.; Perez-Flores, I.; Valero, R.; Rodríguez-Sánchez, B.; García de Viedma, D.; Muñoz, P.; Barrientos, A. Mammalian target of rapamycin signal inhibitors could play a role in the treatment of BK polyomavirus nephritis in renal allograft recipients. Transpl. Infect. Dis. 2011, 13, 584–591. [Google Scholar] [CrossRef]
- Sharma, A.P.; Moussa, M.; Casier, S.; Rehman, F.; Filler, G.; Grimmer, J. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr. Transplant. 2009, 13, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kable, K.; Np, M.N.; Davies, C.D.; Philip, J.O.; Chapman, J.R.; Nankivell, B.J. Clearance of BK Virus Nephropathy by Combination Antiviral Therapy with Intravenous. Transplant. Direct 2017, 3, e142. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, T.; Stein, M.; Reinke, P. ABO desensitization affects cellular immunity and infection control after renal transplantation. Transpl. Int. 2015, 28, 1179–1194. [Google Scholar] [CrossRef]

| mTORi±IVIg Group n = 26 | Control Group n = 17 | Total n = 43 | p-Value | |
|---|---|---|---|---|
| Age (years) | 56.3 (16.3) | 60.5 (10.4) | 58 (14.3) | 0.502 |
| Male n (%) | 22 (85) | 16 (94) | 38 (88) | 0.342 |
| BMI (kg/m2) | 25.8 (3.5) | 26.1 (5.4) | 25.9 (4.3) | 0.691 |
| Diabetes n (%) | 3 (11) | 2 (12) | 5 (12) | 0.982 |
| High blood pressure n (%) | 23 (88) | 15 (88) | 38 (88) | 0.982 |
| Nephropathy n (%) | 0.556 | |||
| 1 (4) | 2 (12) | 3 (7) | |
| 2 (8) | 3 (18) | 5 (12) | |
| 9 (35) | 6 (35) | 15 (30) | |
| 14 (54) | 6 (35) | 20 (46) | |
| Vascular impairment n (%) | ||||
| 0 | 1 (6) | 1 (5) | 0.429 |
| 0 | 0 | 0 | 0.211 |
| Gender of donor: female n (%) | 16 (61) | 11 (65) | 27 (63) | 0.834 |
| Type of donor n (%) | <0.001 | |||
| 5 (19) | 1 (6) | 6 (14) | |
| 6 (23) | 15 (88) | 21 (49) | |
| 15 (58) | 1 (6) | 16 (37) | |
| Age of donor (years) | 53.2 (16.9) | 63.9 (11.4) | 57.5 (15.7) | 0.037 |
| ABO-incompatible n (%) | 4 (15) | 0 | 4 (9) | 0.141 |
| HLA-incompatible n (%) | 4 (15) | 0 | 4 (9) | 0.141 |
| Transplantation rank | 0.238 | |||
| 1 | 21 (81%) | 11 (65%) | 32 (74) | |
| 2 | 5 (19%) | 6 (35%) | 11 (25) | |
| ATG n (%) | 22 (85) | 100 | 39 (91) | 0.140 |
| Mismatch I n (%) | 0.764 | |||
| [0–2] | 11 (42) | 6 (35) | 17 (39) | |
| [3, 4] | 14 (54) | 11 (65) | 25 (58) | |
| Mismatch II n (%) | 0.373 | |||
| [0–2] | 12 (46) | 13 (76) | 25 (58) | |
| [3, 4] | 13 (50) | 4 (23) | 17 (39) | |
| Rituximab n (%) | 8 (31) | 0 | 8 (19) | 0.014 |
| Immunosuppression at medical intervention | ||||
| 25 (96) | 100 | 42 (98) | 0.413 |
| 952.3 (250) | 1062.5 (403) | 994.3 (317) | 0.192 |
| Corticoids n (%) | 25 (96) | 15 (88) | 40 (93) | 0.151 |
| 7 (7.9) | 5.7 (4.2) | 6.5 (6.8) | 0.796 |
| 100% | 100% | 100% | |
| 6.6 [5.7–8.9] | 7.2 [5.4,10.1] | 0.825 | |
| Lymphocytes G/L | 0.7 [0.4–1] | 0.6 [0.5–0.9] | 0.902 | |
| Immunosuppression Modifications in the Control Group | n |
|---|---|
| Lowering tacrolimus (trough level 3–5 ng/mL) | 5 |
| Lowering MMF (500 mg/day) | 1 |
| Lowering MMF (500 mg/day) and tacrolimus (trough level 3–5 ng/mL) * | 4 |
| Lowering MMF (500 mg/day) and tacrolimus and stopping steroids * | 1 |
| Stopping steroids | 3 |
| Stop steroids and lower tacrolimus (trough level 3–5 ng/mL) * | 2 |
| Stop MMF and lower tacrolimus (trough level 3–5 ng/mL) * | 1 |
| Total | 17 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Odd-Ratio [2.5–97.5%] | p-Value | Odd-Ratio [2.5–97.5%] | p-Value | |
| Recipient gender: male | 4.2 [0.61–35.63] | 0.145 | - | - |
| Recipient age | 0.97 [0.92–1.02] | 0.307 | - | - |
| Donor gender: male | 0.22 [0.05–0.87] | 0.035 | 0.10 [0.01–0.59] | 0.02 |
| Living donor | 0.179 | - | - | |
| Rituximab use | 0.34 [0.07–1.73] | 0.188 | 0.36 [0.04–2.7] | 0.33 |
| Donor age | 1.01 [0.96–1.05] | 0.937 | - | - |
| ATG dose | 1.00 [0.99–1.01] | 0.321 | - | - |
| Tac trough concentration at medical intervention | 1.14 [0.86–1.57] | 0.486 | - | - |
| IVIg use | 0.79 [0.44–1.18] | 0.495 | - | - |
| High lymphocyte number at medical intervention | 3.59 [0.62–29.40] | 0.182 | - | - |
| eGFR (mL/min/1.73 m2) at medical intervention | 1.00 [0.96–1.04] | 0.676 | - | - |
| Delay between KT and BKV | 0.90 [0.78–0.98] | 0.066 | 0.88 [0.76–0.96] | 0.019 |
| mTORi versus reduced immunosuppression | 0.10 [0.01–0.48] | 0.033 | 0.11 [0.06–0.9] | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, C.; Jouve, T.; Chevallier, E.; Imerzoukene, F.; Germi, R.; Le Marechal, M.; Truffot, A.; Fiard, G.; Janbon, B.; Giovannini, D.; et al. Conversion to mTOR-Inhibitors Plus IV Immunoglobulins in Kidney-Transplant Recipients with BKV Infection: A Retrospective Comparative Study. J. Clin. Med. 2022, 11, 7292. https://doi.org/10.3390/jcm11247292
Vela C, Jouve T, Chevallier E, Imerzoukene F, Germi R, Le Marechal M, Truffot A, Fiard G, Janbon B, Giovannini D, et al. Conversion to mTOR-Inhibitors Plus IV Immunoglobulins in Kidney-Transplant Recipients with BKV Infection: A Retrospective Comparative Study. Journal of Clinical Medicine. 2022; 11(24):7292. https://doi.org/10.3390/jcm11247292
Chicago/Turabian StyleVela, Carla, Thomas Jouve, Eloi Chevallier, Farida Imerzoukene, Raphaële Germi, Marion Le Marechal, Aurélie Truffot, Gaëlle Fiard, Bénédicte Janbon, Diane Giovannini, and et al. 2022. "Conversion to mTOR-Inhibitors Plus IV Immunoglobulins in Kidney-Transplant Recipients with BKV Infection: A Retrospective Comparative Study" Journal of Clinical Medicine 11, no. 24: 7292. https://doi.org/10.3390/jcm11247292
APA StyleVela, C., Jouve, T., Chevallier, E., Imerzoukene, F., Germi, R., Le Marechal, M., Truffot, A., Fiard, G., Janbon, B., Giovannini, D., Malvezzi, P., Rostaing, L., & Noble, J. (2022). Conversion to mTOR-Inhibitors Plus IV Immunoglobulins in Kidney-Transplant Recipients with BKV Infection: A Retrospective Comparative Study. Journal of Clinical Medicine, 11(24), 7292. https://doi.org/10.3390/jcm11247292







