Donor Noradrenaline Support Is Not Associated with Decreased Survival in Heart Transplant Recipients
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethics
2.2. Patients and Study Design
2.3. Data Collection
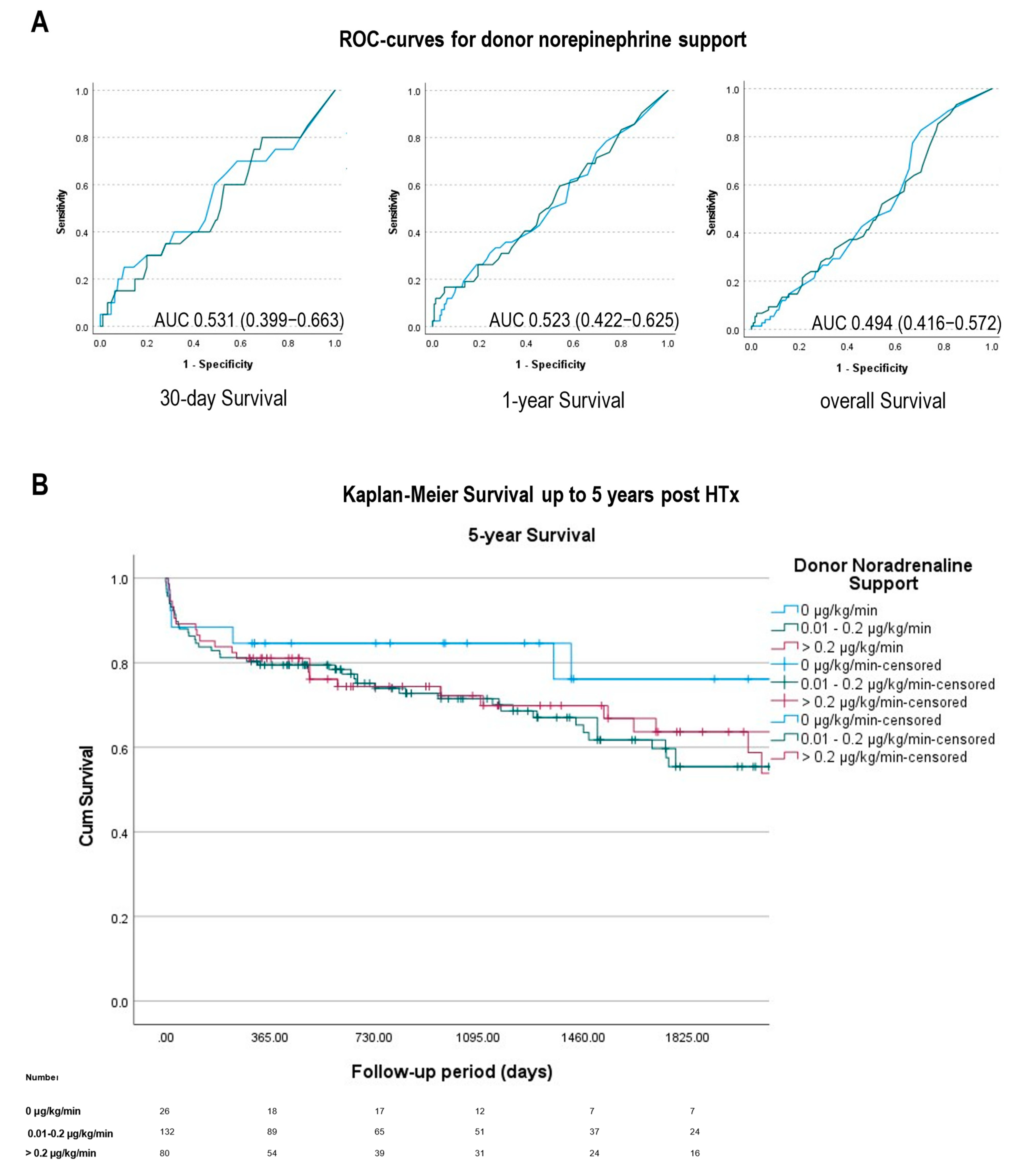
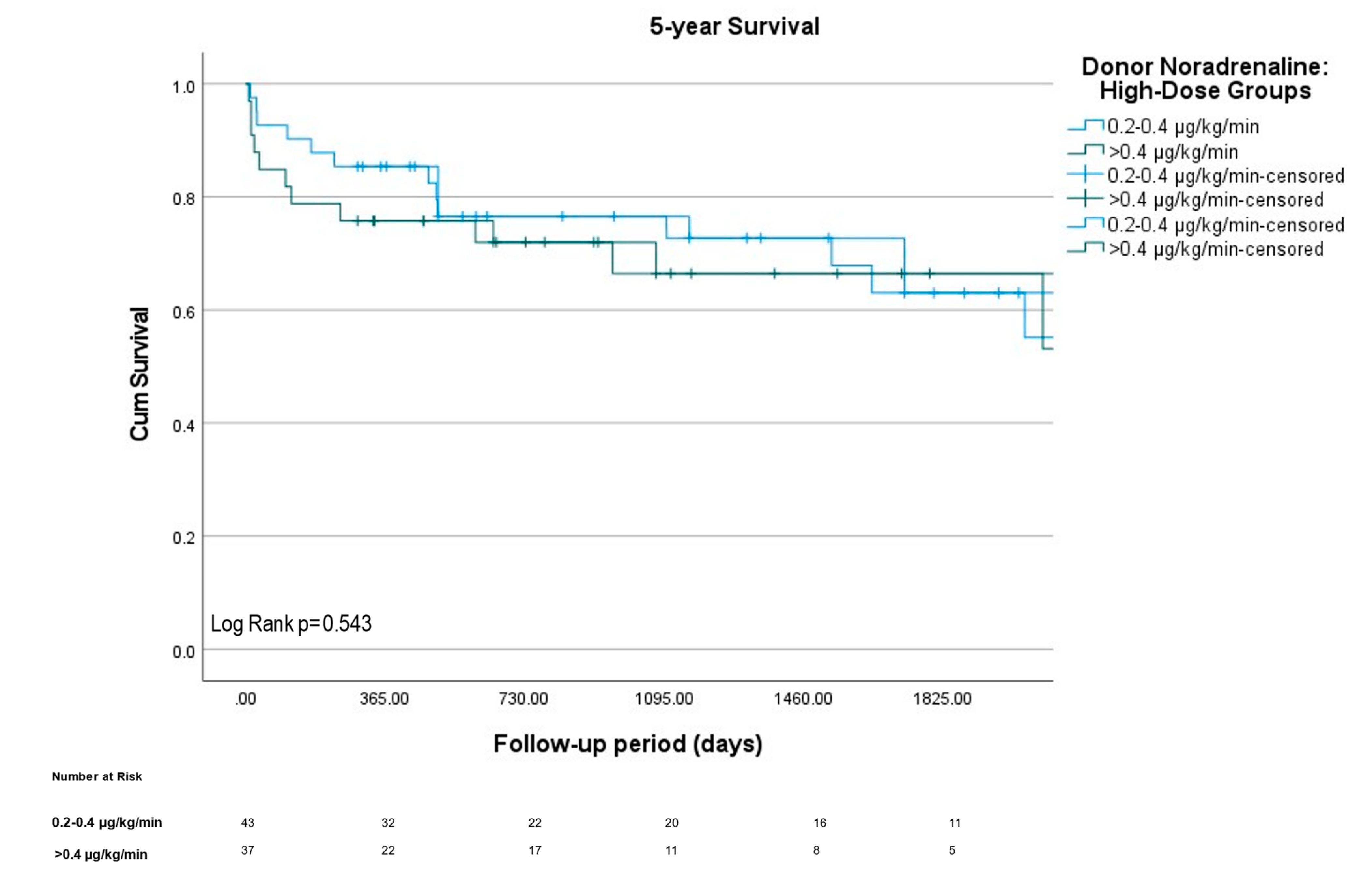
2.4. Statistical Analysis and Figure Making
3. Results
3.1. Recipient Data
3.2. Donor Data
3.3. Perioperative Morbidity
3.4. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTx | Heart Transplantation |
| vs. | versus |
| ECLS | Extracorporeal life support |
| CPR | Cardiopulmonary resuscitation |
| IMC | Intermediate Care Unit |
| ICU | Intensive Care Unit |
References
- Sorabella, R.; Guglielmetti, L.; Kantor, A.; Castillero, E.; Takayama, H.; Schulze, P.; Mancini, D.; Naka, Y.; George, I. Cardiac Donor Risk Factors Predictive of Short-Term Heart Transplant Recipient Mortality: An Analysis of the United Network for Organ Sharing Database. Transplant. Proc. 2015, 47, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.L.; Baroldi, G.; Pieper, G.M.; Clayton, F.C.; Eliot, R.S. Experimental catecholamine-induced myocardial necrosis I. morphology, quantification and regional distribution of acute contraction band lesions. J. Mol. Cell. Cardiol. 1985, 17, 317–338. [Google Scholar] [CrossRef]
- López, S.P.; Hernández, J.O.; Moreno, N.V.; Augusto, D.E.; Menéndez, F.; González, A.A. Brain Death Effects on Catecholamine Levels and Subsequent Cardiac Damage Assessed in Organ Donors. J. Heart Lung Transplant. 2009, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Herijgers, P.; Leunens, V.; Tjandra-Maga, T.B.; Mubagwa, K.; Flameng, W. Changes in Organ Perfusion After Brain Death in The Rat and Its Relation to Circulating Catecholamines1. Transplantation 1996, 62, 330–335. [Google Scholar] [CrossRef]
- Rivinius, R.; Helmschrott, M.; Ruhparwar, A.; Schmack, B.; Darche, F.F.; Thomas, D.; Bruckner, T.; Doesch, A.O.; Katus, H.A.; Ehlermann, P. Elevated pre-transplant pulmonary vascular resistance is associated with early post-transplant atrial fibrillation and mortality. ESC Heart Fail. 2020, 7, 176–187. [Google Scholar] [CrossRef]
- Rizvi, S.-S.A.; Luc, J.G.Y.; Choi, J.H.; Phan, K.; Escrivà, E.M.; Patel, S.; Massey, H.T.; Tchantchaleishvili, V. Outcomes and survival following heart retransplantation for cardiac allograft failure: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2018, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Branco, J.; Dinkhuysen, J.; Junior, J.O.; Pereira, T.; Dinardi, L.; Santos, M.; Dias, R.; Pereira, L.; Stolf, N. Risk Factor Analysis of Late Survival After Heart Transplantation According to Donor Profile: A Multi-Institutional Retrospective Study of 512 Transplants. Transplant. Proc. 2012, 44, 2469–2472. [Google Scholar] [CrossRef]
- Murana, G.; Fiorentino, M.; Gliozzi, G.; Di Marco, L.; Potena, L.; Suarez, S.M.; Pacini, D.; Loforte, A. Donor risk analysis and validation in heart transplants: A single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 860–867. [Google Scholar] [CrossRef]
- Smits, J.M.; De Pauw, M.; de Vries, E.; Rahmel, A.; Meiser, B.; Laufer, G.; Zuckermann, A. Donor scoring system for heart transplantation and the impact on patient survival. J. Heart Lung Transplant. 2012, 31, 387–397. [Google Scholar] [CrossRef]
- Benck, U.; Hoeger, S.; Brinkkoetter, P.T.; Gottmann, U.; Doenmez, D.; Boesebeck, D.; Lauchart, W.; Gummert, J.; Karck, M.; Lehmkuhl, H.B.; et al. Effects of Donor Pre-Treatment with Dopamine on Survival After Heart Transplantation: A Cohort Study of Heart Transplant Recipients Nested in a Randomized Controlled Multicenter Trial. J. Am. Coll. Cardiol. 2011, 58, 1768–1777. [Google Scholar] [CrossRef]
- Kutschmann, M.; Fischer-Fröhlich, C.-L.; Schmidtmann, I.; Bungard, S.; Zeissig, S.R.; Polster, F.; Kirste, G.; Frühauf, N.R. The joint impact of donor and recipient parameters on the outcome of heart transplantation in Germany after graft allocation. Transpl. Int. 2013, 27, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Angleitner, P.; Kaider, A.; Gökler, J.; Moayedifar, R.; Osorio-Jaramillo, E.; Zuckermann, A.; Laufer, G.; Aliabadi-Zuckermann, A. High-dose catecholamine donor support and outcomes after heart transplantation. J. Heart Lung Transplant. 2018, 37, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.; Patel, M.S.; Groat, T.; Jameson, N.E.; Ellis, M.K.M.; Hutchens, M.P.; Niemann, C.U.; Malinoski, D.J.; Sally, M.B. Vasopressor selection during critical care management of brain dead organ donors and the effects on kidney graft function. J. Trauma Acute Care Surg. 2020, 88, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Cuende, N.; Miranda, B.; Cañón, J.F.; Garrido, G.; Matesanz, R. Donor Characteristics Associated with Liver Graft Survival. Transplantation 2005, 79, 1445–1452. [Google Scholar] [CrossRef]
- Braulio, R.; Sanches, M.D.; Junior, A.L.T.; Costa, P.H.N.; Moreira, M.D.C.V.; Rocha, M.A.; De Andrade, S.A.; Gelape, C.L. Associated Clinical and Laboratory Markers of Donor on Allograft Function After Heart Transplant. Braz. J. Cardiovasc. Surg. 2016, 31, 89–97. [Google Scholar] [CrossRef][Green Version]
- D’Aragon, F.; Lamontagne, F.; Cook, D.; Dhanani, S.; Keenan, S.; Chassé, M.; English, S.; Burns, K.E.A.; Frenette, A.J.; Ball, I.; et al. Variability in deceased donor care in Canada: A report of the Canada-DONATE cohort study. Can. J. Anaesth. 2020, 67, 992–1004. [Google Scholar] [CrossRef]
- Oehler, D.; Immohr, M.B.; Erbel-Khurtsidze, S.; Aubin, H.; Bruno, R.R.; Holst, H.T.; Westenfeld, R.; Horn, P.; Kelm, M.; Tudorache, I.; et al. Intracerebral bleeding in donors is associated with reduced short-term to midterm survival of heart transplant recipients. ESC Heart Fail. 2022, 9, 2419–2427. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Petnak, T.; Miao, J.; Qian, Q. Increased short-term and long-term mortality in community- and hospital-acquired hypernatraemia and in patients with delayed serum sodium correction. Int. J. Clin. Pract. 2021, 75, e14590. [Google Scholar] [CrossRef] [PubMed]
- Boeken, U.; Albert, A.; Mehdiani, A.; Petrov, G.; Westenfeld, R.; Saeed, D.; Akhyari, P.; Lichtenberg, A. Impact of Donor Hypernatremia on Outcome after Cardiac Transplantation. Thorac. Cardiovasc. Surg. 2016, 64, ePP96. [Google Scholar] [CrossRef]
- Finger, M.A.; Cipullo, R.; Neto, J.M.R.; Dos Santos, C.C.; Contreras, C.A.; Chaccur, P.; Dinkhuysen, J.J.; de Souza, R.; França, J.I.D.; Lin-Wang, H.T. Donor hypernatremia and smoking addiction contribute to primary graft failure in heart transplantation. Clin. Transplant. 2019, 33, e13693. [Google Scholar] [CrossRef]
- Hoefer, D.; Ruttmann-Ulmer, E.; Smits, J.M.; DeVries, E.; Antretter, H.; Laufer, G. Donor hypo- and hypernatremia are predictors for increased 1-year mortality after cardiac transplantation. Transpl. Int. 2009, 23, 589–593. [Google Scholar] [CrossRef] [PubMed]
| Recipient Variables | All Patients | Gr 1 (0 μg/kg/min) | Gr 2 (0.01–0.2 µg/kg/min) | Gr 3 (>0.2 µg/kg/min) | p | ||
|---|---|---|---|---|---|---|---|
| n = 238 | Total n = 26 | Total n = 132 | Total n = 80 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Age (y) | 58 (50–62) | 60 (42–63) | 58 (50–63) | 59 (53–62) | 0.59 | 0.40 | 0.54 |
| Gender (% male) | 72.2 | 76.9 | 75.0 | 67.5 | >0.99 | 0.46 | 0.27 |
| Height (cm) | 175 (170–180) | 177 (172–180) | 174 (170–180) | 175 (168–180) | 0.58 | 0.33 | 0.48 |
| Weight (kg) | 78 (68–87) | 78 (70–88) | 78 (67–88) | 78 (68–87) | 0.91 | 0.92 | 0.99 |
| Body mass index (kg/m2) | 25 (23–28)) | 24 (22–29) | 25 (23–28) | 26 (23–29) | 0.96 | 0.79 | 0.73 |
| Predicted heart mass ratio (%) | 1.0 (0.9–1.1) | 0.9 (0.8–1.1) | 0.97 (0.87–1.08) | 0.99 (0.91–1.11) | 0.24 | 0.06 | 0.17 |
| Cardiac reoperation (%) | 63.1 | 57.7 | 61.4 | 67.5 | 0.83 | 0.48 | 0.38 |
| High-urgency waiting list (%) | 44.8 | 50.0 | 46.2 | 41.3 | 0.83 | 0.50 | 0.57 |
| Ventricular assist device (%) | 50.6 | 42.3 | 52.3 | 51.3 | 0.40 | 0.50 | 0.89 |
| CPR pre-HTx (%) | 12.9 | 15.4 | 10.6 | 15.2 | 0.82 | >0.99 | 0.38 |
| Diabetes mellitus (%) | 22.6 | 30.8 | 19.8 | 25.3 | 0.30 | 0.61 | 0.39 |
| Arterial Hypertension (%) | 57.1 | 61.5 | 54.5 | 60.8 | 0.53 | >0.99 | 0.39 |
| ICM (%) | 43.2 | 46.2 | 38.6 | 47.5 | 0.52 | >0.99 | 0.20 |
| Laboratory values | |||||||
| Hemoglobin (g/dL) | 12.0 (10.1–13.6) | 12.3 (10.3–13.4) | 12 (10.1–13.6) | 11.8 (9.8–13.7) | 0.95 | 0.94 | 0.84 |
| Creatinine (mg/dL) | 1.2 (1–1.6) | 1.3 (1.1–1.7) | 1.2 (1–1.6) | 1.2 (1–1.5) | 0.33 | 0.67 | 0.56 |
| GFR pre-HTx (mL/min) | 62 (45–82) | 57 (41–77) | 64 (46–85) | 62 (47–79) | 0.47 | 0.46 | 0.93 |
| Bilirubin (mg/dL) | 0.6 (0.4–1.0) | 0.7 (0.4–1.5) | 0.6 (0.4–1) | 0.6 (0.4–0.9) | 0.56 | 0.25 | 0.37 |
| Lactate dehydrogenase (U/L) | 254 (213–314) | 228 (196–306) | 253 (215–292) | 265 (218–365) | 0.47 | 0.12 | 0.26 |
| Sodium (mmol/L) | 138 (136–141) | 138 (136–141) | 138 (136–141) | 138 (136–140) | 0.53 | 0.85 | 0.31 |
| Potassium (mmol/L) | 4.3 (3.9–4.6) | 4.2 (3.8–4.4) | 4.3 (3.93–4.6) | 4.2 (3.9–4.6) | 0.06 | 0.24 | 0.34 |
| Donor Variables | All Patients | Gr 1 (0 μg/kg/min) | Gr 2 (0.01–0.2 µg/kg/min) | Gr 3 (>0.2 µg/kg/min) | p | ||
|---|---|---|---|---|---|---|---|
| n = 238 | Total n = 26 | Total n = 132 | Total n = 80 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Age (y) | 46 (35–53) | 39 (31–49) | 46 (36–54) | 46 (33–52) | 0.06 | 0.14 | 0.56 |
| Gender (% male) | 55.0 | 53.8 | 56.1 | 53.8 | >0.99 | >0.99 | 0.78 |
| Height (cm) | 175 (168–180) | 175 (168–184) | 175 (169–180) | 174 (168–180) | 0.67 | 0.69 | >0.99 |
| Weight (kg) | 80 (70–85) | 79 (65–84) | 80 (70–85) | 80 (70–89) | 0.17 | 0.06 | 0.40 |
| Left ventricular ejection fraction (%) | 60 (55–65) | 61 (55–63) | 60 (57–65) | 60 (52–65) | 0.19 | 0.81 | 0.11 |
| CPR pre-brain death (%) | 28.2 | 26.9 | 28.0 | 28.8 | >0.99 | >0.99 | >0.99 |
| Norepinephrine, peak dose (µg/kg/min) | 0.12 (0.05–0.26) | 0 (0–0) | 0.1 (0.04–0.15) | 0.36 (0.26–0.55) | <0.0001 | <0.0001 | <0.0001 |
| Dobutamin, peak dose (µg/kg/min) * | 4.0 (3.0–4.0) | 4.0 (3.0–4.0) | 4.0 (2.8–4.0) | 4.0 (3.6–4.0) | 0.50 | 0.82 | 0.28 |
| Arterial Hypertension (%) | 50.4 | 42.9 | 50.7 | 52.6 | 0.77 | 0.76 | >0.99 |
| Donor cause of death | |||||||
| ICB (%) | 46.2 | 38.5 | 50.7 | 52.5 | 0.67 | 0.26 | 0.26 |
| Trauma (%) | 22.7 | 26.9 | 43.9 | 21.3 | 0.80 | 0.59 | 0.87 |
| Hypoxic (%) | 16.0 | 30.8 | 22.7 | 17.5 | 0.02 | 0.17 | 0.31 |
| Vascular (%) | 6.3 | 3.8 | 12.1 | 2.5 | 0.48 | >0.99 | 0.09 |
| Other (%) | 8.8 | 0.0 | 9.1 | 6.3 | 0.08 | 0.33 | 0.24 |
| Laboratory values | |||||||
| Hemoglobin (g/dL) | 9.9 (8.2–12) | 10.3 (9–12.7) | 9.8 (8.2–11.8) | 9.8 (8.2–11.8) | 0.43 | 0.31 | 0.69 |
| Sodium (mmol/L) | 149 (144–154) | 144 (141–152) | 149 (144–154) | 150 (144–155) | 0.04 | 0.03 | 0.80 |
| Potassium (mmol/L) | 4.1 (3.8–4.5) | 4.2 (3.8–4.5) | 4 (3.7–4.4) | 4.2 (3.9–4.6) | 0.54 | 0.63 | 0.12 |
| Outcome and Survival | All Patients | Gr 1 (0 μg/kg/min) | Gr 2 (0.01–0.2 µg/kg/min) | Gr 3 (>0.2 µg/kg/min) | p | ||
|---|---|---|---|---|---|---|---|
| n = 238 | Total n = 26 | Total n = 132 | Total n = 80 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Total graft ischemia time (min) | 213 (186–237) | 207 (172–235) | 218 (192–242) | 206 (186–236) | 0.22 | 0.55 | 0.52 |
| Graft cold ischemia time (min) | 148 (126–172) | 145 (119–168) | 154 (133–178) | 143 (126–167) | 0.18 | 0.51 | 0.45 |
| Postoperative hospital stay (d) | 36 (26–51) | 31 (24–40) | 34 (26–53) | 40 (29–54) | 0.18 | 0.13 | 0.74 |
| Postoperative IMC/ICU stay (d) | 16 (10–28) | 14 (11–21) | 15 (9–28) | 19 (10–30) | 0.10 | 0.07 | 0.72 |
| Mechanical ventilation (h) | 67 (25–176) | 47 (14–87) | 68 (25–169) | 95 (33–197) | 0.04 | 0.01 | 0.24 |
| Duration of surgery (min) | 413 (341–506) | 388 (343–493) | 411 (331–513) | 427 (363–490) | 0.80 | 0.38 | 0.34 |
| Blood transfusions (during surgery) | |||||||
| Packed red blood cells, mL | 2970 (1620–4590) | 2970 (1080–4050) | 2700 (1620–4523) | 3240 (1688–5063) | 0.56 | 0.43 | 0.70 |
| Platelets, mL | 880 (660–1540) | 880 (440–1485) | 880 (660–1540) | 880 (495–1540) | 0.14 | 0.25 | 0.78 |
| Fresh frozen plasma, mL | 1000 (0–2000) | 1250 (250–1750) | 1000 (63–2000) | 1250 (0–2438) | 0.57 | 0.22 | 0.37 |
| Blood transfusions (on IMC/ICU) | |||||||
| Packed red blood cells, mL | 1890 (810–4320) | 1350 (743–3038) | 1890 (810–4320) | 2025 (1013–4725) | 0.32 | 0.15 | 0.54 |
| Platelets, mL | 220 (0–1100) | 0 (0–275) | 220 (0–990) | 440 (0–1375) | 0.86 | 0.53 | 0.28 |
| Fresh frozen plasma, mL | 3500 (2000–7000) | 2500 (1500–5250) | 3750 (2000–6750) | 4000 (2000–8188) | 0.30 | 0.22 | 0.80 |
| Postoperative morbidity | |||||||
| Infection/Sepsis (%) | 22.6 | 23.1 | 23.8 | 20.5 | >0.99 | >0.99 | 0.61 |
| Rejection within stay (%) | 6.4 | 3.8 | 6.2 | 7.7 | >0.99 | 0.68 | 0.78 |
| Hemodialysis post-HTx (%) | 57.2 | 44.0 | 58.2 | 60.0 | 0.27 | 0.24 | 0.88 |
| Neurological complications (%) | 15.0 | 11.5 | 16.2 | 14.1 | 0.77 | >0.99 | 0.84 |
| Re-thoracotomy post-HTx (%) | 29.9 | 23.1 | 28.5 | 34.6 | 0.64 | 0.34 | 0.44 |
| ECLS post-HTx (%) | 28.6 | 11.5 | 26.2 | 38.5 | 0.13 | 0.01 | 0.09 |
| Survival | |||||||
| 30-day survival n (%) | 216/238 (90.8) | 23/26 (88.5) | 119/130 (91.5) | 71/79 (89.9) | 0.71 | >0.99 | 0.81 |
| 1-year survival n (%) | 161/203 (79.3) | 18/22 (81.8) | 89/113 (78.8) | 54/68 (79.4) | 0.79 | >0.99 | >0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oehler, D.; Böttger, C.; Immohr, M.B.; Bruno, R.R.; Haschemi, J.; Scheiber, D.; Voß, F.; Horn, P.; Aubin, H.; Tudorache, I.; et al. Donor Noradrenaline Support Is Not Associated with Decreased Survival in Heart Transplant Recipients. J. Clin. Med. 2022, 11, 7271. https://doi.org/10.3390/jcm11247271
Oehler D, Böttger C, Immohr MB, Bruno RR, Haschemi J, Scheiber D, Voß F, Horn P, Aubin H, Tudorache I, et al. Donor Noradrenaline Support Is Not Associated with Decreased Survival in Heart Transplant Recipients. Journal of Clinical Medicine. 2022; 11(24):7271. https://doi.org/10.3390/jcm11247271
Chicago/Turabian StyleOehler, Daniel, Charlotte Böttger, Moritz Benjamin Immohr, Raphael Romano Bruno, Jafer Haschemi, Daniel Scheiber, Fabian Voß, Patrick Horn, Hug Aubin, Igor Tudorache, and et al. 2022. "Donor Noradrenaline Support Is Not Associated with Decreased Survival in Heart Transplant Recipients" Journal of Clinical Medicine 11, no. 24: 7271. https://doi.org/10.3390/jcm11247271
APA StyleOehler, D., Böttger, C., Immohr, M. B., Bruno, R. R., Haschemi, J., Scheiber, D., Voß, F., Horn, P., Aubin, H., Tudorache, I., Westenfeld, R., Akhyari, P., Kelm, M., Lichtenberg, A., & Boeken, U. (2022). Donor Noradrenaline Support Is Not Associated with Decreased Survival in Heart Transplant Recipients. Journal of Clinical Medicine, 11(24), 7271. https://doi.org/10.3390/jcm11247271







