State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review
Abstract
1. Introduction
2. Methodological Approach
2.1. Search Strategy and Selection Criteria
2.2. Study Selection and Data Extraction
3. Results
3.1. Epidemiology
3.2. Biology
3.3. Genetic Factors
3.4. The Risk of Cerebral Hemorrhages and Other Complications
3.5. Treatment
3.6. Technical Background
3.7. Endovascular Techniques
3.8. Endovascular Embolization in Different Clinical Settings
3.8.1. Presurgical and Preradiosurgical Endovascular Embolization
3.8.2. Acute Endovascular Embolization
3.8.3. Curative Endovascular Embolization
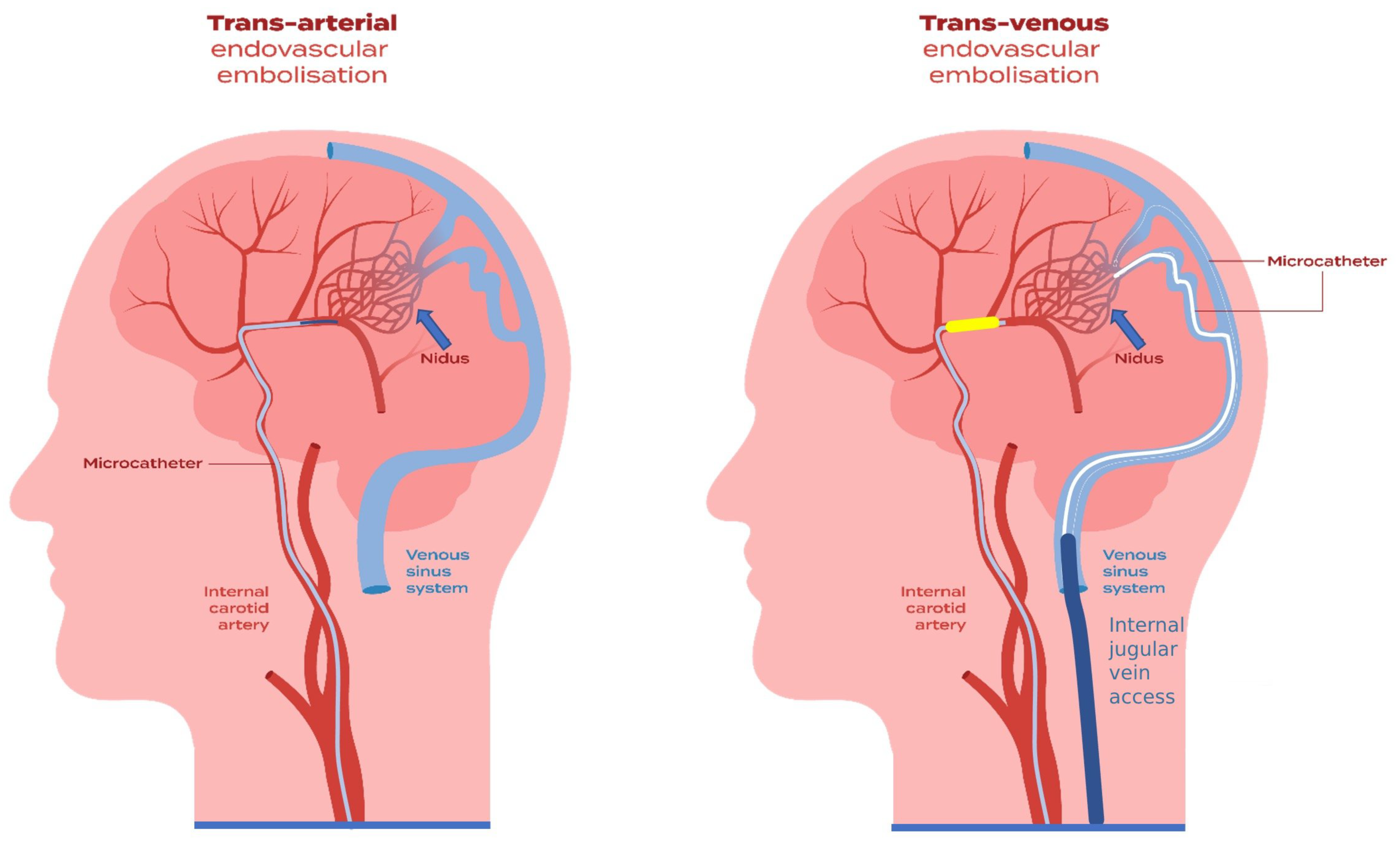
3.9. Transvenous Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solomon, R.A.; Connolly, E.S., Jr. Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2017, 376, 1859–1866. [Google Scholar] [CrossRef]
- Ota, T.; Komiyama, M. Pathogenesis of non-hereditary brain arteriovenous malformation and therapeutic implications. Interv. Neuroradiol. 2020, 26, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Dobson, G.; Prasad, M.; Mukerji, N. De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br. J. Neurosurg. 2018, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Choi, E.-J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Mast, H.; Sciacca, R.R.; Hartmann, A.; Khaw, A.V.; Mohr, J.P.; Sacco, R.L.; Stapf, C. Clinical Outcome After First and Recurrent Hemorrhage in Patients With Untreated Brain Arteriovenous Malformation. Stroke 2006, 37, 1243–1247. [Google Scholar] [CrossRef]
- Germans, M.R.; Sun, W.; Sebök, M.; Keller, A.; Regli, L. Molecular Signature of Brain Arteriovenous Malformation Hemorrhage: A Systematic Review. World Neurosurg. 2021, 157, 143–151. [Google Scholar] [CrossRef]
- Mohr, J.P.; Parides, M.K.; Stapf, C.; Moquete, E.; Moy, C.S.; Overbey, J.R.; Salman, R.A.-S.; Vicaut, E.; Young, W.L.; Houdart, E.; et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet 2013, 383, 614–621. [Google Scholar] [CrossRef]
- Kieu, H.D.; Le, T.D.; Hoang, T.M. Acute spontaneous subdural hematoma secondary to ruptured arteriovenous malformation: A rare entity. Ann. Med. Surg. 2021, 68, 102613. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ding, D.; Derdeyn, C.P.; Lanzino, G.; Friedlander, R.M.; Southerland, A.M.; Lawton, M.T.; Sheehan, J.P. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology 2020, 95, 917–927. [Google Scholar] [CrossRef]
- Jhaveri, A.; Amirabadi, A.; Dirks, P.; Kulkarni, A.; Shroff, M.; Shkumat, N.; Krings, T.; Pereira, V.; Rea, V.; Muthusami, P. Predictive Value of MRI in Diagnosing Brain AVM Recurrence after Angiographically Documented Exclusion in Children. Am. J. Neuroradiol. 2019, 40, 1227–1235. [Google Scholar] [CrossRef]
- Sorenson, T.J.; Brinjikji, W.; Bortolotti, C.; Kaufmann, G.; Lanzino, G. Recurrent Brain Arteriovenous Malformations (AVMs): A Systematic Review. World Neurosurg. 2018, 116, e856–e866. [Google Scholar] [CrossRef]
- Moftakhar, P.; Hauptman, J.S.; Malkasian, D.; Martin, N. Cerebral arteriovenous malformations. Part 2: Physiology. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef]
- Joint Writing Group of the Technology Assessment Committee American Society of Interventional and Therapeutic Neuroradiology; Joint Section on Cerebrovascular Neurosurgery a Section of the American Association of Neurological Surgeons and Congress of Neurological Surgeons; Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology; Atkinson, R.P.; Awad, I.A.; Batjer, H.H.; Dowd, C.F.; Furlan, A.; Giannotta, S.L.; Gomez, C.R.; et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001, 32, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, S.S.; Winkler, E.; Cooke, D.; Su, H. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neurosci. Ther. 2019, 25, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kwon, O.-K.; Bang, J.S.; Lee, H.; Kim, J.E.; Kang, H.-S.; Cho, W.-S.; Oh, C.W. Epidemiology of ruptured brain arteriovenous malformation: A National Cohort Study in Korea. J. Neurosurg. 2019, 130, 1965–1970. [Google Scholar] [CrossRef]
- Al-Shahi, R.; Fang, J.S.Y.; Lewis, S.C.; Warlow, C.P. Prevalence of adults with brain arteriovenous malformations: A community based study in Scotland using capture-recapture analysis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 547–551. [Google Scholar] [CrossRef]
- Brown, R.D.; Wiebers, D.O.; Torner, J.C.; O’Fallon, M.W. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology 1996, 46, 949–952. [Google Scholar] [CrossRef]
- Berman, M.F.; Sciacca, R.R.; Pile-Spellman, J.; Stapf, C.; Connolly, E.S.; Mohr, J.P.; Young, W.L. The Epidemiology of Brain Arteriovenous Malformations. Neurosurgery 2000, 47, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahi, R. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 2001, 124, 1900–1926. [Google Scholar] [CrossRef]
- Laakso, A.; Dashti, R.; Seppänen, J.; Juvela, S.; Väärt, K.; Niemelä, M.; Sankila, R.; Hernesniemi, J.A. Long-term Excess Mortality in 623 Patients with Brain Arteriovenous Malformations. Neurosurgery 2008, 63, 244–255. [Google Scholar] [CrossRef]
- Van Beijnum, J.; Van Der Worp, H.B.; Buis, D.R.; Salman, R.A.-S.; Kappelle, L.J.; Rinkel, G.J.E.; Van Der Sprenkel, J.W.B.; Vandertop, W.P.; Algra, A.; Klijn, C. Treatment of Brain Arteriovenous Malformations. JAMA 2011, 306, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Fults, D.; Kelly, D.L. Natural History of Arteriovenous Malformations of the Brain: A Clinical Study. Neurosurgery 1984, 15, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, W.C.; Ko, N.U.; Lawton, M.T.; Kim, H. Hemorrhage Rates and Risk Factors in the Natural History Course of Brain Arteriovenous Malformations. Transl. Stroke Res. 2014, 5, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Natural history of brain arteriovenous malformations: Systematic review. J. Neurosurg. Sci. 2018, 62, 437–443. [CrossRef]
- Moftakhar, P.; Hauptman, J.S.; Malkasian, D.; Martin, N.A. Cerebral arteriovenous malformations. Part 1: Cellular and molecular biology. Neurosurg. Focus 2009, 26, E10. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Rutledge, W.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; Terbrugge, K.; Kondziolka, D.; Morgan, M.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 2015, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kodama, N.; Sasaki, T.; Matsumoto, M.; Ishikawa, T. Perinidal Dilated Capillary Networks in Cerebral Arteriovenous Malformations. Neurosurgery 2004, 54, 163–170. [Google Scholar] [CrossRef]
- Prado, L.B.D.; Han, C.; Oh, S.P.; Su, H. Recent Advances in Basic Research for Brain Arteriovenous Malformation. Int. J. Mol. Sci. 2019, 20, 5324. [Google Scholar] [CrossRef]
- Ng, I.; Tan, W.-L.; Ng, P.-Y.; Lim, J. Hypoxia inducible factor-1α and expression of vascular endothelial growth factor and its receptors in cerebral arteriovenous malformations. J. Clin. Neurosci. 2005, 12, 794–799. [Google Scholar] [CrossRef]
- Alqadi, M.; Brunozzi, D.; Linninger, A.; Amin-Hanjani, S.; Charbel, F.T.; Alaraj, A. Cerebral arteriovenous malformation venous stenosis is associated with hemodynamic changes at the draining vein-venous sinus junction. Med. Hypotheses 2019, 123, 86–88. [Google Scholar] [CrossRef]
- Mouchtouris, N.; Jabbour, P.M.; Starke, R.M.; Hasan, D.M.; Zanaty, M.; Theofanis, T.; Ding, D.I.; Tjoumakaris, S.; Dumont, A.S.; Ghobrial, G.M.; et al. Biology of Cerebral Arteriovenous Malformations with a Focus on Inflammation. J. Cereb. Blood Flow Metab. 2014, 35, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, R.L.; Lazzaro, M.A.; Castonguay, A.C.; Zaidat, O.O. The Diagnosis and Management of Brain Arteriovenous Malformations. Neurol. Clin. 2013, 31, 749–763. [Google Scholar] [CrossRef]
- Kim, H.; Salman, R.A.-S.; McCulloch, C.E.; Stapf, C.; Young, W.L.; Coinvestigators, F.T.M. Untreated brain arteriovenous malformation: Patient-level meta-analysis of hemorrhage predictors. Neurology 2014, 83, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Saunders, T.; Su, H.; Kim, H.; Akkoc, D.; Saloner, D.A.; Hetts, S.; Hess, C.; Lawton, M.T.; Bollen, A.W.; et al. Silent Intralesional Microhemorrhage as a Risk Factor for Brain Arteriovenous Malformation Rupture. Stroke 2012, 43, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, Y.; Walker, E.J.; Shen, F.; Jun, K.; Oh, S.P.; Degos, V.; Lawton, M.T.; Tihan, T.; Davalos, D.; et al. Reduced Mural Cell Coverage and Impaired Vessel Integrity After Angiogenic Stimulation in the Alk1 -deficient Brain. Arter. Thromb. Vasc. Biol. 2013, 33, 305–310. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, W.; Bollen, A.W.; Lawton, M.T.; Barbaro, N.M.; Dowd, C.F.; Hashimoto, T.; Yang, G.Y.; Young, W.L. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 2008, 62, 1340–1349; discussion 1349–1350. [Google Scholar] [CrossRef]
- Roman, B.L.; Hinck, A.P. ALK1 signaling in development and disease: New paradigms. Cell Mol. Life Sci. 2017, 74, 4539–4560. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, Y.; Lawton, M.T.; Yang, G.-Y.; Barbaro, N.M.; Young, W.L. Coexpression of Angiogenic Factors in Brain Arteriovenous Malformations. Neurosurgery 2005, 56, 1058–1065. [Google Scholar] [CrossRef]
- Hashimoto, T.; Lawton, M.T.; Wen, G.; Yang, G.-Y.; Chaly, T.; Stewart, C.L.; Dressman, H.K.; Barbaro, N.M.; Marchuk, D.A.; Young, W.L. Gene Microarray Analysis of Human Brain Arteriovenous Malformations. Neurosurgery 2004, 54, 410–425. [Google Scholar] [CrossRef]
- Matsubara, S.; Bourdeau, A.; Terbrugge, K.G.; Wallace, C.; Letarte, M. Analysis of Endoglin Expression in Normal Brain Tissue and in Cerebral Arteriovenous Malformations. Stroke 2000, 31, 2653–2660. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Tran, M.N.; Achrol, A.S.; McCulloch, C.E.; Ha, C.; Lind, D.L.; Hashimoto, T.; Zaroff, J.; Lawton, M.T.; Marchuk, D.A.; et al. Polymorphisms in Genes Involved in Inflammatory and Angiogenic Pathways and the Risk of Hemorrhagic Presentation of Brain Arteriovenous Malformations. J. Neurosurg. Anesthesiol. 2004, 35, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Kushamae, M.; Aoki, T.; Yamaguchi, T.; Kitazato, K.; Abekura, Y.; Kawamata, T.; Mizutani, T.; Miyamoto, S.; Takagi, Y. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg. 2019, 126, e1365–e1373. [Google Scholar] [CrossRef] [PubMed]
- Huai, C.; Song, J.; Ma, Z.; Qin, X.; Li, P.; Chen, H.; Zhao, F.; Lu, D.; Song, D.; Mao, Y.; et al. Allelic Variation of the MMP3 Promoter Affects Transcription Activity through the Transcription Factor C-MYB in Human Brain Arteriovenous Malformations. PLoS ONE 2013, 8, e57958. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Birk, H.; Burkhardt, J.-K.; Chen, X.; Yue, J.K.; Guo, D.; Rutledge, W.C.; Lasker, G.F.; Partow, C.; Tihan, T.; et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J. Neurosurg. 2018, 129, 1464–1474. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Jahromi, B.R.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.-R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, S.; Huang, S.; Yoo, J.Y.; Körbelin, J.; Lee, T.J.; Kaur, B.; Dash, P.K.; Chen, P.R.; Kim, E. Selective Endothelial Hyperactivation of Oncogenic KRAS Induces Brain Arteriovenous Malformations in Mice. Ann. Neurol. 2021, 89, 926–941. [Google Scholar] [CrossRef]
- Priemer, D.S.; Vortmeyer, A.O.; Zhang, S.; Chang, H.Y.; Curless, K.L.; Cheng, L. Activating KRAS mutations in arteriovenous malformations of the brain: Frequency and clinicopathologic correlation. Hum. Pathol. 2019, 89, 33–39. [Google Scholar] [CrossRef]
- Hong, T.; Yan, Y.; Li, J.; Radovanovic, I.; Ma, X.; Shao, Y.W.; Yu, J.; Ma, Y.; Zhang, P.; Ling, F.; et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2018, 142, 23–34. [Google Scholar] [CrossRef]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Fish, J.E.; Suarez, C.P.F.; Boudreau, E.; Herman, A.M.; Gutierrez, M.C.; Gustafson, D.; DiStefano, P.V.; Cui, M.; Chen, Z.; De Ruiz, K.B.; et al. Somatic Gain of KRAS Function in the Endothelium Is Sufficient to Cause Vascular Malformations That Require MEK but Not PI3K Signaling. Circ. Res. 2020, 127, 727–743. [Google Scholar] [CrossRef]
- Gao, S.; Nelson, J.; Weinsheimer, S.; Winkler, E.A.; Rutledge, C.; Abla, A.A.; Gupta, N.; Shieh, J.T.; Cooke, D.L.; Hetts, S.W.; et al. Somatic mosaicism in the MAPK pathway in sporadic brain arteriovenous malformation and association with phenotype. J. Neurosurg. 2022, 136, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, S.; Xie, Z.; Zhang, M.; Zhao, H.; Cheng, X.; Zhang, Y.; Niu, Y.; Liu, J.; Zhang, T.J.; et al. Exome-wide Analysis of De Novo and Rare Genetic Variants in Patients With Brain Arteriovenous Malformation. Neurology 2022, 98, e1670–e1678. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.A.; Du, R. Natural history of cerebral arteriovenous malformations: A meta-analysis. J. Neurosurg. 2013, 118, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sahlein, D.H.; Mora, P.; Becske, T.; Huang, P.; Jafar, J.J.; Connolly, E.S.; Nelson, P.K. Features Predictive of Brain Arteriovenous Malformation Hemorrhage. Stroke 2014, 45, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Majumdar, M.; Masoud, H.; Nguyen, T.; Honarmand, A.; Shaibani, A.; Ansari, S.; Tan, L.; Chen, M. Multicenter assessment of morbidity associated with cerebral arteriovenous malformation hemorrhages. J. NeuroInterventional Surg. 2016, 9, 664–668. [Google Scholar] [CrossRef]
- Ferracci, F.-X.; Courthéoux, P.; Borha, A.; Blond, S.; Emery, E. Multimodal management of ruptured cerebral micro-arteriovenous malformations: A retrospective series of 19 cases and a review of the literature. Neurochirurgie 2020, 67, 132–139. [Google Scholar] [CrossRef]
- Stiver, S.I.; Ogilvy, C.S. Micro-arteriovenous Malformations: Significant Hemorrhage from Small Arteriovenous Shunts. Neurosurgery 2000, 46, 811–819. [Google Scholar] [CrossRef]
- Baranoski, J.F.; Grant, R.A.; Hirsch, L.; Visintainer, P.; Gerrard, J.; Günel, M.; Matouk, C.C.; Spencer, D.D.; Bulsara, K.R. Seizure control for intracranial arteriovenous malformations is directly related to treatment modality: A meta-analysis. J. NeuroInterventional Surg. 2013, 6, 684–690. [Google Scholar] [CrossRef]
- Starke, R.M.; Komotar, R.J.; Hwang, B.Y.; Fischer, L.E.; Garrett, M.C.; Otten, M.L.; Connolly, E.S. Treatment guidelines for cerebral arteriovenous malformation microsurgery. Br. J. Neurosurg. 2009, 23, 376–386. [Google Scholar] [CrossRef]
- Guillaumet, G.; Shotar, E.; Clarençon, F.; Sourour, N.-A.; Premat, K.; Lenck, S.; Dupont, S.; Jacquens, A.; Degos, V.; Boeken, T.; et al. Incidence and risk factors of epilepsy following brain arteriovenous malformation rupture in adult patients. J. Neurol. 2022, 269, 6342–6353. [Google Scholar] [CrossRef]
- Smajda, S.; Ciccio, G.; Fahed, R.; Robert, T.; Botta, D.; Redjem, H.; Desilles, J.-P.; Mazighi, M.; Zuber, K.; Escalard, S.; et al. Visual Field Defect Before and After Endovascular Treatment of Occipital Arteriovenous Malformations. Neurosurgery 2020, 87, E663–E671. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, A.R.; Thines, L.; Willinsky, R.A.; Terbrugge, K.G.; Schwartz, M.L.; Tymianski, M.; Wallace, M.C. Multidisciplinary care of occipital arteriovenous malformations: Effect on nonhemorrhagic headache, vision, and outcome in a series of 135 patients. J. Neurosurg. 2010, 113, 742–748. [Google Scholar] [CrossRef]
- Weiskrantz, L.; Warrington, E.K.; Sanders, M.D.; Marshall, J. Visual Capacity in the Hemianopic Field Following a Restricted Occipital Ablation. Brain 1974, 97, 709–728. [Google Scholar] [CrossRef]
- Yang, W.; Porras, J.L.; Philadelphia, E.; Law, J.; Garzon-Muvdi, T.; Caplan, J.M.; Colby, G.P.; Coon, A.L.; Tamargo, R.J.; Huang, J. Treatment decision for occipital arteriovenous malformations (AVMs) to achieve hemorrhagic control while maximizing visual preservation: Our experience and review of literature. J. Clin. Neurosci. 2018, 48, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tawk, R.G.; Tummala, R.P.; Memon, M.Z.; Siddiqui, A.H.; Hopkins, L.N.; Levy, E.I. Utility of Pharmacologic Provocative Neurological Testing Before Embolization of Occipital Lobe Arteriovenous Malformations. World Neurosurg. 2011, 76, 276–281. [Google Scholar] [CrossRef]
- O’Donnell, J.M.; Morgan, M.K.; Manuguerra, M.; Bervini, D.; Assaad, N. Patient functional outcomes and quality of life after surgery for unruptured brain arteriovenous malformation. Acta Neurochir. 2021, 163, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Saatci, I.; Geyik, S.; Yavuz, K.; Cekirge, H.S. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: Long-term results in 350 consecutive patients with completed endovascular treatment course. J. Neurosurg. 2011, 115, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Ponce, F.A. A 3-tier classification of cerebral arteriovenous malformations. J. Neurosurg. 2011, 114, 842–849. [Google Scholar] [CrossRef]
- Lawton, M.T.; Kim, H.; McCulloch, C.E.; Mikhak, B.; Young, W.L. A Supplementary Grading Scale for Selecting Patients With Brain Arteriovenous Malformations for Surgery. Neurosurgery 2010, 66, 702–713. [Google Scholar] [CrossRef]
- Wegner, R.E.; Oysul, K.; Pollock, B.E.; Sirin, S.; Kondziolka, D.; Niranjan, A.; Lunsford, L.D.; Flickinger, J.C. A Modified Radiosurgery-Based Arteriovenous Malformation Grading Scale and Its Correlation With Outcomes. Int. J. Radiat. Oncol. 2011, 79, 1147–1150. [Google Scholar] [CrossRef]
- Starke, R.M.; Yen, C.-P.; Ding, D.; Sheehan, J.P. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: Analysis of 1012 treated patients. J. Neurosurg. 2013, 119, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; He, H.; Liu, P.; Gao, D.; Chen, Y.; Sun, S.; Liu, A.; Li, Y.; Jin, H. Radiosurgery-Based AVM Scale Is Proposed for Combined Embolization and Gamma Knife Surgery for Brain Arteriovenous Malformations. Front. Neurol. 2021, 12, 647167. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Dumont, T.; Kan, P.; Snyder, K.; Hopkins, L.; Siddiqui, A. A proposed grading system for endovascular treatment of cerebral arteriovenous malformations: Buffalo score. Surg. Neurol. Int. 2015, 6, 3. [Google Scholar] [CrossRef]
- Feliciano, C.E.; de León-Berra, R.; Hernández-Gaitán, M.S.; Rodríguez-Mercado, R. A proposal for a new arteriovenous malformation grading scale for neuroendovascular procedures and literature review. P R Health Sci. J. 2010, 29, 117–120. [Google Scholar]
- Lopes, D.K.; Moftakhar, R.; Straus, D.; Munich, S.A.; Chaus, F.; Kaszuba, M.C. Arteriovenous malformation embocure score: AVMES. J. NeuroInterventional Surg. 2015, 8, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Adeeb, N.; Moore, J.M.; Motiei-Langroudi, R.; Griessenauer, C.J.; Patel, A.S.; Ogilvy, C.S.; Thomas, A.J. Validity assessment of grading scales predicting complications from embolization of cerebral arteriovenous malformations. Clin. Neurol. Neurosurg. 2016, 151, 102–107. [Google Scholar] [CrossRef]
- Orosz, P.; Vadász, Á.; Veres, D.S.; Berentei, Z.; Gubucz, I.; Nardai, S.; Kis, B.; Szikora, I. Living with a Brain AVM: A Quality of Life Assessment. Trends Cerebrovasc. Surg. Interv. 2021, 132, 71–76. [Google Scholar] [CrossRef]
- De Leacy, R.; Ansari, S.A.; Schirmer, C.M.; Cooke, D.L.; Prestigiacomo, C.J.; Bulsara, K.R.; Hetts, S.W. Endovascular treatment in the multimodality management of brain arteriovenous malformations: Report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J. NeuroInterventional Surg. 2022, 14, 1118–1124. [Google Scholar] [CrossRef]
- Mohr, J.P.; Overbey, J.R.; Hartmann, A.; von Kummer, R.; Salman, R.A.-S.; Kim, H.; van der Worp, H.B.; Parides, M.K.; Stefani, M.A.; Houdart, E.; et al. Medical management with interventional therapy versus medical management alone for unruptured brain arteriovenous malformations (ARUBA): Final follow-up of a multicentre, non-blinded, randomised controlled trial. Lancet Neurol. 2020, 19, 573–581. [Google Scholar] [CrossRef]
- Magro, E.; Gentric, J.-C.; Darsaut, T.E.; Ziegler, D.; Msi; Bojanowski, M.W.; Raymond, J. Responses to ARUBA: A systematic review and critical analysis for the design of future arteriovenous malformation trials. J. Neurosurg. 2017, 126, 486–494. [Google Scholar] [CrossRef]
- Salman, R.A.-S.; White, P.M.; Counsell, C.E.; du Plessis, J.; van Beijnum, J.; Josephson, C.B.; Wilkinson, T.; Wedderburn, C.J.; Chandy, Z.; George, E.J.S.; et al. Outcome After Conservative Management or Intervention for Unruptured Brain Arteriovenous Malformations. JAMA 2014, 311, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Sun, Y.; Chang, Q.; You, W.; Liu, P.; Lv, X.; Li, Y. Endovascular treatment of cerebellar arteriovenous malformations: A single-center experience of 75 consecutive patients. Neurol. India 2020, 68, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; Shotar, E.; Premat, K.; Boch, A.-L.; Delaitre, M.; Borius, P.-Y.; Nouet, A.; Lenck, S.; Talbi, A.; Bessar, A.; et al. Safety and Effectiveness of First-line Endovascular Management of Low-Grade Brain Arteriovenous Malformations. Clin. Neuroradiol. 2022, 7, 1–11. [Google Scholar] [CrossRef]
- Hou, K.; Xu, K.; Qu, L.; Li, G.; Guo, Y.; Yu, J. Angiographic Evaluation and Endovascular Treatment Considerations of Brain Arteriovenous Malformations With a Transdural Blood Supply: A Single-Center Experience. Front. Neurol. 2021, 11, 603256. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Z.; Jiang, C.; Li, Y.; Yang, X.; Zhang, Y.; Zhang, N. Complication risk of endovascular embolization for cerebral arteriovenous malformation. Eur. J. Radiol. 2011, 80, 776–779. [Google Scholar] [CrossRef]
- Baharvahdat, H.; Blanc, R.; Fahed, R.; Smajda, S.; Ciccio, G.; Desilles, J.-P.; Redjem, H.; Escalard, S.; Mazighi, M.; Chauvet, D.; et al. Endovascular Treatment for Low-Grade (Spetzler-Martin I–II) Brain Arteriovenous Malformations. Am. J. Neuroradiol. 2019, 40, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Baharvahdat, H.; Blanc, R.; Fahed, R.; Pooyan, A.; Mowla, A.; Escalard, S.; Delvoye, F.; Desilles, J.P.; Redjem, H.; Ciccio, G.; et al. Endovascular treatment as the main approach for Spetzler–Martin grade III brain arteriovenous malformations. J. NeuroInterventional Surg. 2020, 13, 241–246. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Bai, W.; Li, T.; Hui, F.; Jiang, W.-J.; Xue, J. Safety and Efficacy of Transvenous Embolization of Ruptured Brain Arteriovenous Malformations as a Last Resort: A Prospective Single-Arm Study. Am. J. Neuroradiol. 2019, 40, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Poncyljusz, W.; Sawicki, M.; Lubkowska, K.; Rać, M. Early outcomes and periprocedural complications of transarterial embolization of brain arteriovenous malformations with Onyx®. Neurol. i Neurochir. Polska 2017, 51, 277–285. [Google Scholar] [CrossRef]
- Van Rooij, W.; Jacobs, S.; Sluzewski, M.; Beute, G.; van der Pol, B. Endovascular Treatment of Ruptured Brain AVMs in the Acute Phase of Hemorrhage. Am. J. Neuroradiol. 2012, 33, 1162–1166. [Google Scholar] [CrossRef]
- Iosif, C.; de Lucena, A.F.; Abreu-Mattos, L.G.; Ala, V.H.E.; El-Ghanam, A.; Saleme, S.; Caire, F.; Mounayer, C. Curative endovascular treatment for low-grade Spetzler-Martin brain arteriovenous malformations: A single-center prospective study. J. NeuroInterventional Surg. 2019, 11, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Januel, A.-C.; Herbreteau, D.; Barreau, X.; Drouineau, J.; Berge, J.; Sourour, N.; Cognard, C. Endovascular treatment of brain arteriovenous malformations using onyx: Results of a prospective, multicenter study. J. Neuroradiol. 2009, 36, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Cognard, C.; Herbreteau, D.; Fransen, H.; Van Rooij, W.J.; Boccardi, E.; Beltramello, A.; Sourour, N.; Kupcs, K.; Biondi, A.; et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: Results of a prospective, multicentre study (BRAVO). Eur. Radiol. 2013, 23, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Katsaridis, V.; Papagiannaki, C.; Aimar, E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 2008, 50, 589–597. [Google Scholar] [CrossRef]
- Panagiotopoulos, V.; Gizewski, E.; Asgari, S.; Regel, J.; Forsting, M.; Wanke, I. Embolization of Intracranial Arteriovenous Malformations with Ethylene-Vinyl Alcohol Copolymer (Onyx). Am. J. Neuroradiol. 2008, 30, 99–106. [Google Scholar] [CrossRef]
- Jahan, R.; Murayama, Y.; Gobin, Y.P.; Duckwiler, G.R.; Vinters, H.V.; Viñuela, F. Embolization of Arteriovenous Malformations with Onyx: Clinicopathological Experience in 23 Patients. Neurosurgery 2001, 48, 984–997. [Google Scholar] [CrossRef]
- Triano, M.J.; Lara-Reyna, J.; Schupper, A.J.; Yaeger, K.A. Embolic Agents and Microcatheters for Endovascular Treatment of Cerebral Arteriovenous Malformations. World Neurosurg. 2020, 141, 383–388. [Google Scholar] [CrossRef]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2021, 32, 25–38. [Google Scholar] [CrossRef]
- Hu, J.; Albadawi, H.; Chong, B.W.; Deipolyi, A.; Sheth, R.A.; Khademhosseini, A.; Oklu, R. Advances in Biomaterials and Technologies for Vascular Embolization. Adv. Mater. 2019, 31, e1901071. [Google Scholar] [CrossRef]
- Tamura, G.; Kato, N.; Yamazaki, T.; Akutsu, Y.; Hosoo, H.; Kasuya, H.; Sonobe, M. Endovascular Embolization of Brain Arteriovenous Malformations with Eudragit-E. Neurol. Med.-Chirurgica 2015, 55, 253–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellis, J.A.; Lavine, S.D. Role of Embolization for Cerebral Arteriovenous Malformations. Methodist DeBakey Cardiovasc. J. 2014, 10, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Sussman, E.S.; Mayercik, V.; Steinberg, G.K.; Do, H.M.; Heit, J.J. Initial experience with the Scepter Mini dual-lumen balloon for transophthalmic artery embolization of anterior cranial fossa dural arteriovenous fistulae. J. NeuroInterventional Surg. 2020, 12, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Durst, C.R.; Starke, R.M.; Gaughen, J.; Evans, A.J. A method for complete angiographic obliteration of a brain arteriovenous malformation in a single session through a single pedicle. J. Clin. Neurosci. 2014, 22, 391–395. [Google Scholar] [CrossRef]
- Waldeck, S.; Chapot, R.; von Falck, C.; Froelich, M.F.; Brockmann, M.; Overhoff, D. First Experience in the Control of the Venous Side of the Brain AVM. J. Clin. Med. 2021, 10, 5771. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, R. Basics and Principles in the Application of Onyx LD Liquid Embolic System in the Endovascular Treatment of Cerebral Arteriovenous Malformations. Interv. Neuroradiol. 2005, 11, 131–140. [Google Scholar] [CrossRef]
- Brosnan, C.; Amoo, M.; Javadpour, M. Preoperative embolisation of brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurg. Rev. 2022, 45, 2051–2063. [Google Scholar] [CrossRef]
- Chapot, R.; Stracke, P.; Velasco, A.; Nordmeyer, H.; Heddier, M.; Stauder, M.; Schooss, P.; Mosimann, P.J. The Pressure Cooker Technique for the treatment of brain AVMs. J. Neuroradiol. 2014, 41, 87–91. [Google Scholar] [CrossRef]
- Cekirge, H.S.; Saatci, I. Multiplug flow control technique as a novel transarterial curative approach for the endovascular treatment of cerebrovascular malformations. BMJ Case Rep. 2021, 14, e017418. [Google Scholar] [CrossRef]
- Luzzi, S.; Del Maestro, M.; Bongetta, D.; Zoia, C.; Giordano, A.V.; Trovarelli, D.; Dehcordi, S.R.; Galzio, R. Onyx Embolization Before the Surgical Treatment of Grade III Spetzler-Martin Brain Arteriovenous Malformations: Single-Center Experience and Technical Nuances. World Neurosurg. 2018, 116, e340–e353. [Google Scholar] [CrossRef]
- Donzelli, G.F.; Nelson, J.; McCoy, D.; McCulloch, C.E.; Hetts, S.W.; Amans, M.R.; Dowd, C.F.; Halbach, V.V.; Higashida, R.T.; Lawton, M.T.; et al. The effect of preoperative embolization and flow dynamics on resection of brain arteriovenous malformations. J. Neurosurg. 2020, 132, 1836–1844. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Z.; Zhang, Y.; Wang, Y.; Lai, L. Preradiosurgery embolization in reducing the postoperative hemorrhage rate for patients with cerebral arteriovenous malformations: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, S.; Izumi, T.; Satow, T.; Srivatanakul, K.; Matsumoto, Y.; Terada, T.; Matsumaru, Y.; Kiyosue, H. J-REAL study investigators Effectiveness of Preradiosurgical Embolization with NBCA for Arteriovenous Malformations—Retrospective Outcome Analysis in a Japanese Registry of 73 Patients (J-REAL study). Neurointervention 2017, 12, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Gobin, Y.P.; Laurent, A.; Merienne, L.; Schlienger, M.; Aymard, A.; Houdart, E.; Casasco, A.; Lefkopoulos, D.; George, B.; Merland, J.J. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J. Neurosurg. 1996, 85, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, S.; Negoro, M.; Okamoto, T.; Kobayashi, T.; Kida, Y.; Tanaka, T.; Yoshida, J. Embolisation of cerebral arteriovenous malformations to assure successful subsequent radiosurgery. J. Clin. Neurosci. 2000, 7, 82–85. [Google Scholar] [CrossRef]
- Oermann, E.K.; Ding, D.; Yen, C.-P.; Starke, R.M.; Bederson, J.B.; Kondziolka, D.; Sheehan, J.P. Effect of Prior Embolization on Cerebral Arteriovenous Malformation Radiosurgery Outcomes. Neurosurgery 2015, 77, 406–417. [Google Scholar] [CrossRef]
- Marks, M.P.; Marcellus, M.L.; Santarelli, J.; Dodd, R.L.; Do, H.M.; Chang, S.D.; Adler, J.R.; Mlynash, M.; Steinberg, G.K. Embolization Followed by Radiosurgery for the Treatment of Brain Arteriovenous Malformations (AVMs). World Neurosurg. 2016, 99, 471–476. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ding, D.; Lee, C.-C.; Kearns, K.N.; Pomeraniec, I.J.; Cifarelli, C.P.; Arsanious, D.E.; Liscak, R.; Hanuska, J.; Williams, B.J.; et al. Stereotactic Radiosurgery With Versus Without Embolization for Brain Arteriovenous Malformations. Neurosurgery 2021, 88, 313–321. [Google Scholar] [CrossRef]
- Russell, D.; Peck, T.; Ding, D.; Chen, C.-J.; Taylor, D.G.; Starke, R.M.; Lee, C.-C.; Sheehan, J.P. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: A systematic review and meta-analysis. J. Neurosurg. 2018, 128, 1338–1348. [Google Scholar] [CrossRef]
- Derdeyn, C.P.; Zipfel, G.J.; Albuquerque, F.C.; Cooke, D.L.; Feldmann, E.; Sheehan, J.P.; Torner, J.C. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e200–e224. [Google Scholar] [CrossRef]
- Alexander, M.D.; Cooke, D.L.; Hallam, D.K.; Kim, H.; Hetts, S.W.; Ghodke, B.V. Less can be more: Targeted embolization of aneurysms associated with arteriovenous malformations unsuitable for surgical resection. Interv. Neuroradiol. 2016, 22, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, H.; Li, Y.; Tian, Z. Target Embolization of Associated Aneurysms in Ruptured Arteriovenous Malformations. World Neurosurg. 2017, 101, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, W.J.; Jacobs, S.; Sluzewski, M.; van der Pol, B.; Beute, G.N.; Sprengers, M.E. Curative embolization of brain arteriovenous malformations with onyx: Patient selection, embolization technique, and results. AJNR Am. J. Neuroradiol. 2012, 33, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, F.; Gory, B.; Pelissou-Guyotat, I.; Guyotat, J.; Riva, R.; Dailler, F.; Turjman, F. Ruptured brain arteriovenous malformations associated with aneurysms: Safety and efficacy of selective embolization in the acute phase of hemorrhage. Neuroradiology 2014, 56, 763–769. [Google Scholar] [CrossRef]
- Bendjilali, N.; Nelson, J.; Weinsheimer, S.; Sidney, S.; Zaroff, J.G.; Hetts, S.; Segal, M.; Pawlikowska, L.; McCulloch, C.E.; Young, W.L.; et al. Common variants on 9p21.3 are associated with brain arteriovenous malformations with accompanying arterial aneurysms. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1280–1283. [Google Scholar] [CrossRef]
- Meisel, H.J.; Mansmann, U.; Alvarez, H.; Rodesch, G.; Brock, M.; Lasjaunias, P. Cerebral Arteriovenous Malformations and Associated Aneurysms: Analysis of 305 Cases from a Series of 662 Patients. Neurosurgery 2000, 46, 793–802. [Google Scholar] [CrossRef]
- Turjman, F.; Massoud, T.F.; Viñuela, F.; Sayre, J.W.; Guglielmi, G.; Duckwiler, G. Aneurysms related to cerebral arteriovenous malformations: Superselective angiographic assessment in 58 patients. AJNR Am. J. Neuroradiol. 1994, 15, 1601–1605. [Google Scholar]
- Koyanagi, M.; Mosimann, P.J.; Nordmeyer, H.; Heddier, M.; Krause, J.; Narata, A.-P.; El Serwi, A.; Stracke, C.P.; Chapot, R. The transvenous retrograde pressure cooker technique for the curative embolization of high-grade brain arteriovenous malformations. J. NeuroInterventional Surg. 2020, 13, 637–641. [Google Scholar] [CrossRef]
- Crowley, R.W.; Ducruet, A.F.; Kalani, M.Y.S.; Kim, L.J.; Albuquerque, F.C.; McDougall, C.G. Neurological morbidity and mortality associated with the endovascular treatment of cerebral arteriovenous malformations before and during the Onyx era. J. Neurosurg. 2015, 122, 1492–1497. [Google Scholar] [CrossRef]
- Wu, E.M.; El Ahmadieh, T.Y.; McDougall, C.M.; Aoun, S.G.; Mehta, N.; Neeley, O.J.; Plitt, A.; Ban, V.S.; Sillero, R.; White, J.A.; et al. Embolization of brain arteriovenous malformations with intent to cure: A systematic review. J. Neurosurg. 2020, 132, 388–399. [Google Scholar] [CrossRef]
- Massoud, T.F.; Hademenos, G.J. Transvenous Retrograde Nidus Sclerotherapy under Controlled Hypotension (TRENSH): A Newly Proposed Treatment for Brain Arteriovenous Malformations-Concepts and Rationale. Neurosurgery 1999, 45, 351–366. [Google Scholar] [CrossRef]
- Kessler, I.; Riva, R.; Ruggiero, M.; Manisor, M.; Al-Khawaldeh, M.; Mounayer, C. Successful Transvenous Embolization of Brain Arteriovenous Malformations Using Onyx in Five Consecutive Patients. Neurosurgery 2011, 69, 184–193. [Google Scholar] [CrossRef]
- Iosif, C.; Mendes, G.A.C.; Saleme, S.; Ponomarjova, S.; Silveira, E.P.; Caire, F.; Mounayer, C. Endovascular transvenous cure for ruptured brain arteriovenous malformations in complex cases with high Spetzler-Martin grades. J. Neurosurg. 2015, 122, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Mullan, S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J. Neurosurg. 1994, 80, 606–616. [Google Scholar] [CrossRef]
- Mendes, G.A.C.; Kalani, M.Y.S.; Iosif, C.; Lucena, A.F.; Carvalho, R.; Saleme, S.; Mounayer, C. Transvenous Curative Embolization of Cerebral Arteriovenous Malformations: A Prospective Cohort Study. Neurosurgery 2017, 83, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Griessenauer, C.J.; Wipplinger, C.; Jabbour, P.; Asl, M.K.; Rahbarian, F.; Mortazavi, A. Adenosine-induced transient circulatory arrest in transvenous embolization of cerebral arteriovenous malformations. Neuroradiol. J. 2021, 34, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.; Filho, J.A.A.; Gilbert, C.E.; Rafie, A.N.; Saleme, S.; Rouchaud, A.; Mounayer, C. Selective arterial temporary flow arrest with balloons during transvenous embolization for the treatment of brain arteriovenous malformations: A feasibility study with MRI-monitored adverse events. J. NeuroInterventional Surg. 2022, 14, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
| Graded Feature | Points Assigned | |
|---|---|---|
| Buffalo score (Dumont and colleagues) [74] | ||
| Number of article pedicles | 1 or 2 | 1 |
| 3 or 4 | 2 | |
| 5 or more | 3 | |
| Diameter of arterial pedicles | Most > 1 mm | 0 |
| Most ≤ 1 mm | 1 | |
| Nidus location | Noneloquent | 0 |
| Eloquent | 1 | |
| AVM neuroendovascular grade (Feliciano and colleagues) [75] | ||
| Number of feeding vessels | Less than 3 | 1 |
| 3 or more and less than 6 | 2 | |
| More than 6 | 3 | |
| Eloquence of adjacent areas | Noneloquent | 0 |
| Eloquent | 1 | |
| Presence of an arteriovenous fistula | No | 0 |
| Yes | 1 | |
| AVM embocure score (AVMES) (Lopes and colleagues) [76] | ||
| Size of AVM nidus | Smaller than 3 cm | 1 |
| Larger than 3 cm but smaller than 6 cm | 2 | |
| Larger than 6 cm | 3 | |
| Number of arterial pedicles feeding AVM | 1 or 3 | 1 |
| 4 or 6 | 2 | |
| More than 6 | 3 | |
| Number of draining veins | 1 or 3 | 1 |
| 4 or 6 | 2 | |
| More than 6 | 3 | |
| Vascular eloquence * | Noneloquent | 0 |
| Eloquent | 1 | |
| Author (Year) | No. of Patients | No. of Embolizations | Agent Used | SM Grade | Mean Age (Years) | Presenting Symptoms | No. of Patients with Associated Aneurysms | Follow-Up Period | Mortality | Complications | Outcome | Obliteration Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saatci et al. (2011) [67] | 350 | 607 | Onyx (n = 308) Onyx with n-BCA (n = 42) patients | I (n = 52) II (n = 106) III (n = 99) IV (n = 69) V (n = 24) | 34 | Hemorrhage (n = 163) Seizure (n = 118) Headache (n = 27) Neurological signs (n = 29) Incidental (n = 13) | NS | Mean 47 months | 1.4% | Permanent morbidity rate—7.4% | mRS score > 2 (n = 15) | 51% |
| Sun et al. (2020) [83] | 75 | 97 | Onyx nBCA | I (n = 28) II (n = 31) III (n = 11) IV (n = 5) I–III (n = 70) IV–V (n = 5) | 37.7 ± 15.5 | Hemorrhage (n = 61) Nonhemorrhagic (n = 14) | 44 | Mean 73 months | 2.7% | 13.13% | 0–3 mRS score (n = 61) | 42.7% |
| Lv et al. (2010) [86] | 147 * | 220 | Onyx (n = 76) nBCA (n = 144) | I (n = 5) II (n = 20) III (n = 54) IV (n = 44) V (n = 24) | 27.5 ± 11.1 | Hemorrhage (n = 69) Seizure (n = 43) Headache (n = 21) Focal neurologic deficit (n = 11) Incidental (n = 3) | 7 | NS | 0% | 4.8% | 0–2 mRS score (n = 141) 3 mRS score (n = 6) | 19.7% |
| Baharvahdat et al. (2019) [87] | 224 | 319 sessions + 8 patients had more than 3 sessions | Onyx nBCA | I (n = 71) II (n = 153) | 37.8 ± 16 | Hemorrhage (n = 136) Seizure (n = 42) Incidental (n = 37) Other (n = 9) | 62 | Mean 9.7 ± 11.9 months | 0.4% | Permanent neurological deficit in 5% of patients | mRS ≤ 2 (n = 179) 13 patients had a worse mRS score compared with their preoperative status | 92% |
| Baharvahdat et al. (2020) [88] | 65 | 102 + 6 patients had more than 3 sessions | Onyx nBCA | III | 40.5 ± 14 | Hemorrhage (n = 40) Seizure (n = 9) Isolated neurological deficit (n = 4) Incidental (n = 5) | 37 | Mean 12 months | 3% | Permanent neurological deficit in 6.2% of patients | 0–2 mRS score (n = 55) 3-5 mRS score (n = 10) Eight patients (12.3%) experienced worsening of mRS after embolization | 87.7% |
| He et al. (2019) [89] | 21 | NS | Onyx | I (n = 3) II (n = 4) III (n = 11) IV (n = 3) | 29.9 | IC hematoma and IV hemorrhage (n = 15) IC hematoma (n = 6) SA hemorrhage (n = 1) IV hemorrhage (n = 1) | 9 | Mean 6 months | 4.8% | Morbidity rate 4.8% | 0–2 mRS score (n = 19) | 76.2% |
| Poncyljusz et al. (2017) [90] | 54 | 108 | Onyx | I (n = 5) II (n = 19) III (n = 22) IV (n = 7) V (n = 1) | 42.6 ± 15.4 | Hemorrhage (n = 27) Headaches (n = 12) Seizures (n = 7) Focal neurological deficits (n = 2) Incidental (n = 6) | 8 | Mean 33.3 months | 1.8% | Morbidity rate 5.6% | SM score: I–II (n = 24) III–V (n = 30) | 46.3% |
| van Rooij et al. (2012) [91] | 23 | NS | Onyx | NS | 42 | AVM-related hemorrhagic stroke | 9 | 21 months | 1 patient died | None | No repeated hemorrhage during the 21 months of follow-up 3 patients were dependent in a nursing home and 19 patients were functioning independently | 57% |
| Iosif et al. (2019) [92] | 73 | 84 | Onyx | I (n = 16) II (n = 57) | 40.5 ± 17.8 | Rupture (n = 44) Epileptic seizure (n = 6) Headache (n = 16) Neurologic deficit (n = 2) Incidental (n = 5) | 14 | 6 months | 0 | Procedure-related morbidity was 2.7% | 90.5% of the patients were independent in their everyday lives (mRS score 0–2) | Total occlusion of the nidus in all but one case |
| Pierot et al. (2009) [93] | 50 | 149 | Onyx (n = 116) Glue (n = 20) Onyx and glue (n = 13) | NS | 34.8 | Hemorrhage (n = 22) Seizure (n = 16) Headache (n = 6) Progressive neurological deficit (n = 2) Incidental (n = 4) | NS | 1 month | 2% | Morbidity 8% | Out of the 44 patients with incomplete occlusion after embolization, 37 were proposed for radiosurgery | Percentage of occlusion was 100% in four cases (8.3%), 80 to 99% in 27 cases (56.3%), 60 to 79% in 8 cases (16.7%), and less than 60% in 9 cases (18.7%) |
| Pierot et al. (2013) [94] | 117 | 237 | Onyx (n = 187) Onyx and glue (n = 37) Onyx and coils (n = 1) Glue (n = 12) | I (n = 20) II (n = 44) III (n = 28) IV (n = 24) V (n = 1) | 42.6 ± 13.6 | Hemorrhage (n = 40) Seizure (n = 33) Headache (n = 20) Progressive neurological deficit (n = 11) Incidental (n = 13) | 32 | NS | 4.3% | Morbidity rate 5.1% Permanent deficits 6.0% | 65/79 surviving patients needed complementary treatment | 100% occlusion in 23/61 patients with AVMs < 3 cm and 4/53 patients with AVMs ≥ 3 cm |
| Katsaridis et al. (2008) [95] | 101 | 219 | Onyx | I (n = 7) II (n = 18) III (n = 39) IV (n = 33) V (n = 4) | 38.8 | Hemorrhage (n = 40) Seizure (n = 26) Headache (n = 17) Neurological deficit (n = 17) Incidental (n = 1) | NS | NS | 3% | Morbidity rate 8% | 48.5% are still undergoing the course of endovascular treatment with additional embolization sessions to be performed. | Total occlusion—(53.9%); near-total occlusion (34.6%) |
| Panagiotopoulos et al. (2009) [96] | 82 | 119 | Onyx | I-II (n = 59) III (n = 16) IV–V (n = 7) | 44.2 | IC hemorrhage (n = 37) Seizures (n = 18) Neurologic deficits (n = 8) Headaches (n = 9) Incidental symptoms (n = 10) | NS | 6 months | 2.4% | Morbidity rate 3.8% | An average of 75% volume reduction | 24.4% with an average of 75% (range: 30–100%) volume reduction |
| Jahan et al. (2001) [97] | 23 | 33 | Onyx | I (n = 2) II ( n = 5) III (n = 11) IV (n = 5) | 40 | IC hemorrhage (n = 6) Seizure (n = 9) Headache (n = 4) Neurological deficit (n = 4) | NS | NS | 0% | 4% | Average 63% reduction in AVM volume | NS |
| NCT Number | Study Type | Recruitment Status | Follow-Up | Estimated Enrollment | Intervention | Objective |
|---|---|---|---|---|---|---|
| NCT02180958 | Observational | Completed | 36 months | 140 | Endovascular embolization | To assess the safety and efficacy of Onyx treatment for cAVM |
| NCT02602990 | Observational | Completed | 6 months | 50 | Endovascular embolization | To assess the safety and efficacy of SQUID™ liquid embolic agent |
| NCT04136860 | Observational | Recruiting | 5 years | 1000 | Conservative, microsurgical resection, embolization, embolization + radiosurgery, single-stage hybrid surgery (embolization–resection) | To assess the neurological function prognosis, occlusion rate, and complications |
| NCT02098252 | Interventional | Recruiting | 10 years | 1000 | Procedure: neurosurgery Radiation: radiation therapy Procedure: embolization | To assess whether:
|
| NCT03691870 | Interventional | Recruiting | 3 (+/−1) months post-treatment | 76 | Transarterial embolization (TAE) Transvenous embolization (TVE) | The experimental treatment is an attempt to completely occlude arteriovenous malformations using venous catheterization and retrograde ethyl vinyl alcohol (EVOH) injection during the final session |
| NCT03209804 | Interventional | Completed | 12 months | 519 |
| To assess the clinical benefits and risks of hybrid operating techniques in the management of cerebral arteriovenous malformations (AVMs) |
| NCT00389181 | Interventional | Completed | 5 years | 226 | Comparator: any combination of surgery, endovascular embolization, or radiotherapyExperimental: medical management | To determine whether medical management is better than invasive therapy in improving the long-term outcome of patients with unruptured brain arteriovenous malformations |
| NCT03774017 | Interventional | Unknown | 12 months | 1200 | Experimental: one-stage hybrid operationComparator: traditional microsurgical operation | To validate the benefits and risks of a one-stage hybrid operation in the treatment of complex bAVMs |
| NCT03031873 | Interventional | Recruiting | N/A | 12 | MRI perfusion imaging to evaluate the evolution of progressive obliteration of the AVM nidus | Evaluation of susceptibility-weighted MRI and 4D-time-resolved magnetic resonance angiography in bAVMs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zawadzki, M. State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review. J. Clin. Med. 2022, 11, 7208. https://doi.org/10.3390/jcm11237208
Pinkiewicz M, Pinkiewicz M, Walecki J, Zawadzki M. State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review. Journal of Clinical Medicine. 2022; 11(23):7208. https://doi.org/10.3390/jcm11237208
Chicago/Turabian StylePinkiewicz, Miłosz, Mateusz Pinkiewicz, Jerzy Walecki, and Michał Zawadzki. 2022. "State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review" Journal of Clinical Medicine 11, no. 23: 7208. https://doi.org/10.3390/jcm11237208
APA StylePinkiewicz, M., Pinkiewicz, M., Walecki, J., & Zawadzki, M. (2022). State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review. Journal of Clinical Medicine, 11(23), 7208. https://doi.org/10.3390/jcm11237208






