Effects of Neoadjuvant Radiotherapy on Survival in Patients with Stage IIIA-N2 Non-Small-Cell Lung Cancer Following Pneumonectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patients
2.3. Statistical Analysis
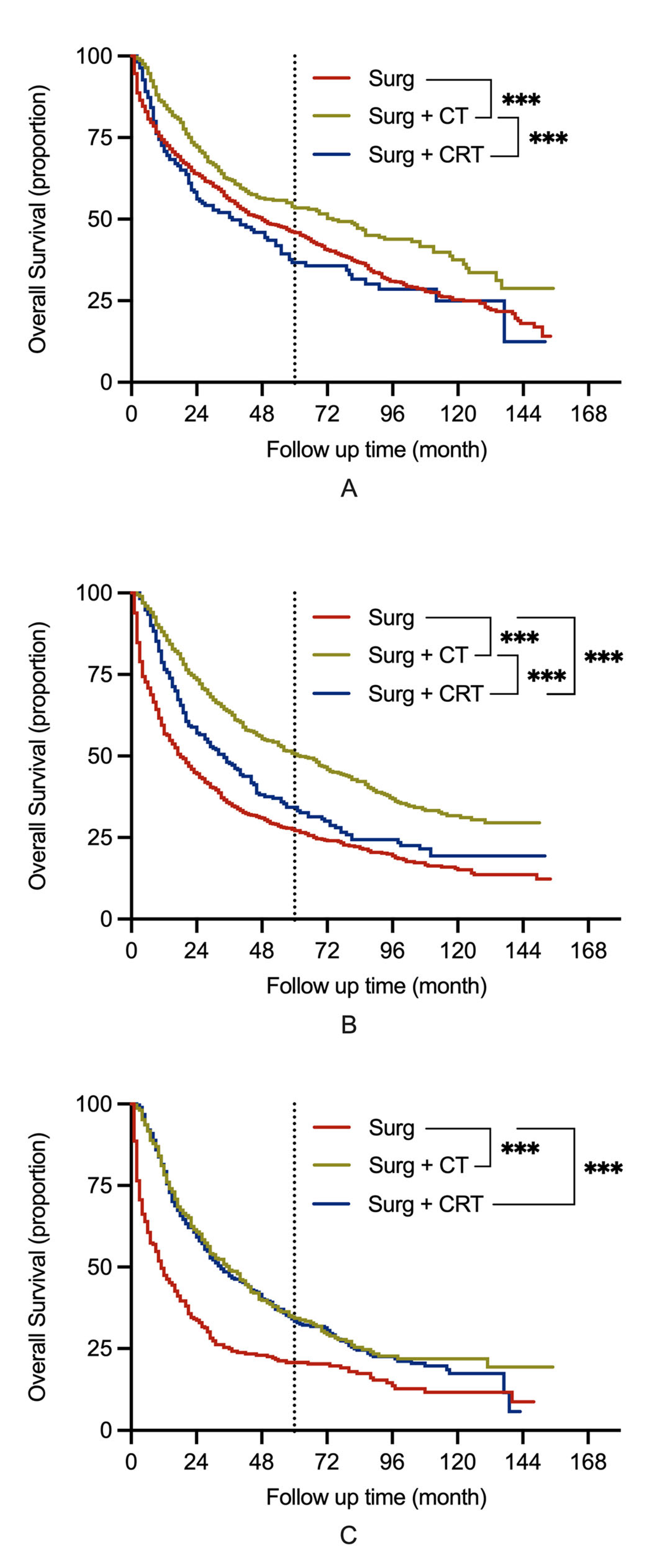
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | adenocarcinoma |
| ASMD | absolute standardized mean difference |
| CI | confidence interval |
| CRT | chemoradiotherapy |
| CT | chemotherapy |
| HR | hazard ratio |
| NART | neoadjuvant radiotherapy |
| NSCLC | non–small cell lung cancer |
| OS | overall survival |
| PORT | postoperative radiotherapy |
| PSM | propensity score-matched |
| RCS | restricted cubic splines |
| RT | radiotherapy |
| SCC | squamous cell carcinoma |
| SD | standardized difference |
| SEER | Surveillance, Epidemiology, and End Results |
References
- Graham, E.A.; Singer, J.J. Landmark article Oct 28, 1933. Successful removal of an entire lung for carcinoma of the bronchus. JAMA 1984, 251, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, S.; Xue, Q.; Tan, F.; Gao, Y.; Mao, Y.; Wang, D.; Zhao, J.; Li, Y.; Wang, F.; et al. Development of a nomogram for predicting the operative mortality of patients who underwent pneumonectomy for lung cancer: A population-based analysis. Transl. Lung Cancer Res. 2021, 10, 381–391. [Google Scholar] [CrossRef]
- Thomas, P.A.; Berbis, J.; Baste, J.M.; Le Pimpec-Barthes, F.; Tronc, F.; Falcoz, P.E.; Dahan, M.; Loundou, A. Pneumonectomy for lung cancer: Contemporary national early morbidity and mortality outcomes. J. Thorac. Cardiovasc. Surg. 2015, 149, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.E.; Rauma, V.H.; Sihvo, E.I.; Räsänen, J.V.; Ilonen, I.K.; Salo, J.A. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: A propensity-matched analysis of long-term results, survival and quality of life. J. Thorac. Dis. 2015, 7, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Evison, M.; Clive, A.; Castle, L.; Powell, H.; Thomas, R.; Buttery, R.; Masani, V.; Harden, S.; West, D.; Woolhouse, I. Resectable Clinical N2 Non-Small Cell Lung Cancer; What Is the Optimal Treatment Strategy? An Update by the British Thoracic Society Lung Cancer Specialist Advisory Group. J. Thorac. Oncol. 2017, 12, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Adizie, J.B.; Khakwani, A.; Beckett, P.; Navani, N.; West, D.; Woolhouse, I.; Harden, S.V. Stage III Non-small Cell Lung Cancer Management in England. Clin. Oncol. 2019, 31, 688–696. [Google Scholar] [CrossRef]
- Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [CrossRef]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar]
- Couñago, F.; Rodriguez de Dios, N.; Montemuiño, S.; Jové-Teixidó, J.; Martin, M.; Calvo-Crespo, P.; López-Mata, M.; Samper-Ots, M.P.; López-Guerra, J.L.; García-Cañibano, T.; et al. Neoadjuvant treatment followed by surgery versus definitive chemoradiation in stage IIIA-N2 non-small-cell lung cancer: A multi-institutional study by the oncologic group for the study of lung cancer (Spanish Radiation Oncology Society). Lung Cancer 2018, 118, 119–127. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Rosell, R.; De Lena, M.; Riggi, M.; Hurteloup, P.; Mahe, M.A. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 695–701. [Google Scholar]
- Pless, M.; Stupp, R.; Ris, H.B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, N.; Xu, Y.; Yang, G. Effects of Postoperative Radiotherapy on Survival of Patients with Stage IIIA Resected Non–Small Cell Lung Cancer: Analysis of the SEER Database. J. Natl. Compr. Cancer Netw. 2020, 18, 718. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Liang, L.; Xie, S.; Wang, C. The impact of order with radiation therapy in stage IIIA pathologic N2 NSCLC patients: A population-based study. BMC Cancer 2020, 20, 809. [Google Scholar] [CrossRef] [PubMed]
- Lally, B.E.; Zelterman, D.; Colasanto, J.M.; Haffty, B.G.; Detterbeck, F.C.; Wilson, L.D. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2006, 24, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Burdett, S.; Rydzewska, L.; Tierney, J.; Fisher, D.; Parmar, M.K.; Arriagada, R.; Pignon, J.P.; Le Pechoux, C. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst. Rev. 2016, 10, Cd002142. [Google Scholar]
- Wisnivesky, J.P.; Halm, E.A.; Bonomi, M.; Smith, C.; Mhango, G.; Bagiella, E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer 2012, 118, 4478–4485. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Hui, Z.; Men, Y.; Hu, C.; Kang, J.; Sun, X.; Bi, N.; Zhou, Z.; Liang, J.; Lv, J.; Feng, Q.; et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1178–1185. [Google Scholar] [CrossRef]
- Corso, C.D.; Rutter, C.E.; Wilson, L.D.; Kim, A.W.; Decker, R.H.; Husain, Z.A. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. J. Thorac. Oncol. 2015, 10, 148–155. [Google Scholar] [CrossRef]
- Yendamuri, S.; Groman, A.; Miller, A.; Demmy, T.; Hennon, M.; Dexter, E.; Picone, A.; Nwogu, C.; Dy, G.K. Risk and benefit of neoadjuvant therapy among patients undergoing resection for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2018, 53, 656–663. [Google Scholar] [CrossRef]
- Glatzer, M.; Leskow, P.; Caparrotti, F.; Elicin, O.; Furrer, M.; Gambazzi, F.; Dutly, A.; Gelpke, H.; Guckenberger, M.; Heuberger, J.; et al. Stage III N2 non-small cell lung cancer treatment: Decision-making among surgeons and radiation oncologists. Transl. Lung. Cancer. Res. 2021, 10, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mayer, M.; Kann, R.; Weder, W.; Zouhair, A.; Betticher, D.C.; Roth, A.D.; Stahel, R.A.; Majno, S.B.; Peters, S.; et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: A multicentre phase II trial. Lancet Oncol. 2009, 10, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; Saldaña, J.; Linares, J.; Ruffinelli, J.C.; Palmero, R.; Navarro, A.; Arnaiz, M.D.; Brao, I.; Aso, S.; Padrones, S.; et al. Geriatric assessment may help decision-making in elderly patients with inoperable, locally advanced non-small-cell lung cancer. Br. J. Cancer 2018, 118, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Du, Y.; Hu, Z.; Liang, J.; Bian, Y.; Chen, Z.; Sui, Q.; Zhan, C.; Li, M.; Guo, W. Long-term outcomes following neoadjuvant or adjuvant chemoradiotherapy for stage I-IIIA non-small cell lung cancer: A propensity-matched analysis. J. Thorac. Dis. 2020, 12, 3043–3056. [Google Scholar] [CrossRef]
- Putora, P.M.; Leskow, P.; McDonald, F.; Batchelor, T.; Evison, M. International guidelines on stage III N2 nonsmall cell lung cancer: Surgery or radiotherapy? ERJ Open Res. 2020, 6, 00159–2019. [Google Scholar] [CrossRef]
- Gao, F.; Li, N.; Xu, Y.; Yang, G. Evaluation of Postoperative Radiotherapy Effect on Survival of Resected Stage III-N2 Non-small Cell Lung Cancer Patients. Front. Oncol. 2020, 10, 1135. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Lerouge, D.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; Dansin, E.; Paumier, A.; et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): An open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 104–114. [Google Scholar] [CrossRef]
- Ahern, E.; Solomon, B.J.; Hui, R.; Pavlakis, N.; O’Byrne, K.; Hughes, B.G.M. Neoadjuvant immunotherapy for non-small cell lung cancer: Right drugs, right patient, right time? J. Immunother. Cancer 2021, 9, e002248. [Google Scholar] [CrossRef]
- Roller, J.F.; Veeramachaneni, N.K.; Zhang, J. Exploring the Evolving Scope of Neoadjuvant Immunotherapy in NSCLC. Cancers 2022, 14, 741. [Google Scholar] [CrossRef]
- De Marinis, F.; Ciardiello, F.; Baas, P.; Crinò, L.; Giaccone, G.; Grossi, F.; Hellmann, M.D.; Mok, T.S.K.; Lena, H.; Paz-Ares, L.; et al. 30 Immunotherapy in advanced NSCLC-from the ‘tsunami’ of therapeutic knowledge to a clinical practice algorithm: Results from an international expert panel meeting of the Italian Association of Thoracic Oncology (AIOT). ESMO Open 2018, 3, e000298. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Rubio, X.; Montemuiño, S.; Jiménez, U.; Luna, J.; Cardeña, A.; Mezquita, L.; Martín, M.; Couñago, F. Management of Resectable Stage III-N2 Non-Small-Cell Lung Cancer (NSCLC) in the Age of Immunotherapy. Cancers 2021, 13, 4811. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet. Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef]



| Characteristics | Treatment (N = 4308) | p | ||
|---|---|---|---|---|
| Surg N = 2156 (%) | CT N = 1380 (%) | CRT N = 772 (%) | ||
| Age (Year) | 65.56 | 61.49 | 59.73 | <0.001 †,* |
| Sex | ||||
| Male | 1433 (66.5) | 877 (63.6) | 518 (67.1) | 0.132 |
| Female | 723 (33.5) | 503 (36.4) | 254 (32.9) | |
| Race | ||||
| Black | 173 (8.0) | 111 (8.0) | 66 (8.5) | 0.428 |
| White | 1877 (87.1) | 1181 (85.6) | 666 (86.3) | |
| Others | 106 (4.9) | 88 (6.4) | 40 (5.2) | |
| Laterality | ||||
| Left | 1202 (55.8) | 831 (60.2) | 465 (60.2) | 0.012 * |
| Right | 954 (44.2) | 549 (39.8) | 307 (39.8) | |
| Pathological type | ||||
| SCC | 1127 (52.3) | 722 (52.3) | 410 (53.1) | 0.787 |
| ADC | 900 (41.7) | 565 (40.9) | 320 (41.5) | |
| Others | 129 (6.0) | 93 (6.7) | 42 (5.4) | |
| Grade | ||||
| I/II | 987 (45.8) | 566 (41.0) | 276 (35.8) | <0.001 * |
| III | 1038 (48.1) | 748 (54.2) | 390 (50.5) | |
| Unknown | 131 (6.1) | 66 (4.8) | 106 (13.7) | |
| T | ||||
| 1 | 348 (16.1) | 113 (8.2) | 43 (5.6) | <0.001 * |
| 2 | 1250 (58.0) | 836 (60.6) | 356 (46.1) | |
| 3 | 239 (11.1) | 197 (14.3) | 150 (19.4) | |
| 4 | 319 (14.8) | 234 (17.0) | 223 (28.9) | |
| N | ||||
| 0 | 1119 (51.9) | 372 (27.0) | 165 (21.4) | <0.001 * |
| 1 | 751 (34.8) | 694 (50.3) | 230 (29.8) | |
| 2 | 286 (13.3) | 314 (22.8) | 377 (48.8) | |
| Stage | ||||
| I | 902 (41.8) | 253 (18.3) | 69 (8.9) | <0.001 * |
| II | 627 (29.1) | 546 (39.6) | 149 (19.3) | |
| III | 627 (29.1) | 581 (42.1) | 554 (71.8) | |
| The only cancer | ||||
| Yes | 1429 (66.3) | 985 (71.4) | 590 (76.4) | <0.001 * |
| No | 727 (33.7) | 395 (28.6) | 182 (23.6) | |
| Marital status | ||||
| Single | 265 (12.3) | 174 (12.6) | 79 (10.2) | <0.001 * |
| Married | 1287 (59.7) | 876 (63.5) | 527 (68.3) | |
| Divorced | 285 (13.2) | 185 (13.4) | 100 (13.0) | |
| Widowed | 257 (11.9) | 10 (7.9) | 46 (6.0) | |
| Unknown | 62 (2.9) | 36 (2.6) | 20 (2.6) | |
| Surgery | ||||
| Pneumonectomy | 2088 (96.8) | 1325 (96.0) | 741 (96.0) | 0.330 |
| Extended pneumonectomy | 68 (3.2) | 55 (4.0) | 31 (4.0) | |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI | p | HR | 95.0% CI | p | |
| Age | 1.026 | 1.022–1.029 | <0.001 * | 1.026 | 1.022–1.030 | <0.001 * |
| Sex | ||||||
| Male | Ref. | Ref. | ||||
| Female | 0.775 | 0.716–0.839 | <0.001 * | 0.750 | 0.689–0.816 | <0.001 * |
| Race | ||||||
| Black | Ref. | |||||
| White | 1.060 | 0.923–1.216 | 0.410 | |||
| Others | 0.927 | 0.750–1.146 | 0.486 | |||
| Laterality | ||||||
| Left | Ref. | Ref. | ||||
| Right | 1.183 | 1.098–1.273 | <0.001* | 1.220 | 1.132–1.314 | <0.001 * |
| Pathological type | ||||||
| SCC | Ref. | Ref. | ||||
| ADC | 1.002 | 0.928–1.082 | 0.950 | 1.096 | 1.011–1.189 | 0.027 * |
| Others | 1.200 | 1.033–1.394 | 0.017* | 1.140 | 0.977–1.329 | 0.097 |
| Grade | ||||||
| I/II | Ref. | Ref. | ||||
| III | 1.222 | 1.132–1.319 | <0.001 * | 1.190 | 1.099–1.288 | <0.001 * |
| Unknown | 1.097 | 0.941–1.277 | 0.236 | 1.044 | 0.894–1.220 | 0.585 |
| T | ||||||
| 1 | Ref. | Ref. | ||||
| 2 | 1.101 | 0.977–1.242 | 0.116 | 1.082 | 0.957–1.225 | 0.208 |
| 3 | 1.266 | 1.093–1.468 | 0.002 * | 1.322 | 1.134–1.541 | <0.001 * |
| 4 | 1.388 | 1.208–1.594 | <0.001 * | 1.352 | 1.171–1.560 | <0.001 * |
| N | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 1.198 | 1.100–1.304 | <0.001 * | 1.402 | 1.282–1.532 | <0.001 * |
| 2 | 1.421 | 1.291–1.564 | <0.001 * | 1.798 | 1.618–1.998 | <0.001 * |
| The only cancer | ||||||
| Yes | Ref. | |||||
| No | 0.976 | 0.903–1.056 | 0.550 | |||
| Marital status | ||||||
| Single | Ref. | Ref. | ||||
| Married | 0.924 | 0.823–1.037 | 0.181 | 0.802 | 0.713–0.902 | <0.001 * |
| Divorced | 0.962 | 0.832–1.113 | 0.606 | 0.881 | 0.760–1.020 | 0.089 |
| Widowed | 1.213 | 1.039–1.415 | 0.014 * | 0.950 | 0.807–1.120 | 0.544 |
| Unknown | 0.794 | 0.609–1.035 | 0.088 | 0.782 | 0.600–1.021 | 0.070 |
| Surgery | ||||||
| Pneumonectomy | Ref. | |||||
| Extended pneumonectomy | 1.375 | 1.139–1.659 | 0.001 * | 1.421 | 1.175–1.718 | <0.001 * |
| Treatment | ||||||
| Surgery | Ref. | Ref. | ||||
| Chemotherapy | 0.622 | 0.570–0.678 | <0.001 * | 0.573 | 0.523–0.628 | <0.001 * |
| Chemoradiotherapy | 0.863 | 0.782–0.953 | 0.003 | 0.733 | 0.656–0.819 | <0.001 * |
| Characteristics | Before Matching (N = 647) | After Matching (N = 440) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT N = 302 (%) | CRT N = 345 (%) | p | SD (%) | CT N = 220 (%) | CRT N = 220 (%) | p | SD (%) | ASMD (%) | |
| Age (year) | 60.57 | 59.02 | 0.04 †,* | 16.2 | 59.11 | 60.75 | 0.060 †,* | 17.9 | 1.7 |
| Sex | 0.369 | 7.1 | 0.920 | 1.0 | 0.6 | ||||
| Male | 184 (60.9) | 222 (64.3) | 143 (65.0) | 144 (65.5) | |||||
| Female | 118 (39.1) | 123 (35.7) | 77 (35.0) | 76 (34.5) | |||||
| Laterality | 0.371 | 7.1 | 0.772 | 2.8 | 0.7 | ||||
| Left | 176 (58.3) | 189 (54.8) | 128 (58.2) | 125 (56.8) | |||||
| Right | 126 (41.7) | 156 (45.2) | 92 (41.8) | 95 (43.2) | |||||
| Pathological type | 0.088 | 17.3 | 0.794 | 6.5 | 1.9 | ||||
| SCC | 147 (48.7) | 166 (48.1) | 110 (50.0) | 104 (47.3) | |||||
| ADC | 131 (43.4) | 165 (47.8) | 101 (45.9) | 108 (49.1) | |||||
| Others | 24 (7.9) | 14 (4.1) | 9 (4.1) | 8 (8) | |||||
| Grade | <0.001 * | 33.6 | 0.972 | 2.3 | 0.5 | ||||
| I/II | 122 (40.4) | 127 (36.8) | 91 (41.4) | 91(41.4) | |||||
| III | 164 (54.3) | 165 (47.8) | 120 (54.5) | 119 (54.1) | |||||
| Unknown | 16 (5.3) | 53 (15.4) | 9 (4.1) | 10 (4.5) | |||||
| T | 0.335 | 14.6 | 0.981 | 4.0 | 1.3 | ||||
| 1 | 24 (7.9) | 24 (7.0) | 15 (6.8) | 16 (7.3) | |||||
| 2 | 179 (59.3) | 184 (53.3) | 125 (56.8) | 121 (55.0) | |||||
| 3 | 34 (11.3) | 49 (14.2) | 28 (12.7) | 30 (13.6) | |||||
| 4 | 65 (21.5) | 88 (25.5) | 52 (23.6) | 53 (24.1) | |||||
| Marital status | 0.144 | 11.5 | 0.614 | 4.8 | 0.7 | ||||
| Married | 191 (63.2) | 237 (68.7) | 143 (65.0) | 148 (67.3) | |||||
| Others | 111 (36.8) | 108 (31.3) | 77 (35.0) | 72 (32.7) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.; Li, R.; Han, J.; Yue, W.; Tian, H. Effects of Neoadjuvant Radiotherapy on Survival in Patients with Stage IIIA-N2 Non-Small-Cell Lung Cancer Following Pneumonectomy. J. Clin. Med. 2022, 11, 7188. https://doi.org/10.3390/jcm11237188
Qu C, Li R, Han J, Yue W, Tian H. Effects of Neoadjuvant Radiotherapy on Survival in Patients with Stage IIIA-N2 Non-Small-Cell Lung Cancer Following Pneumonectomy. Journal of Clinical Medicine. 2022; 11(23):7188. https://doi.org/10.3390/jcm11237188
Chicago/Turabian StyleQu, Chenghao, Rongyang Li, Jingyi Han, Weiming Yue, and Hui Tian. 2022. "Effects of Neoadjuvant Radiotherapy on Survival in Patients with Stage IIIA-N2 Non-Small-Cell Lung Cancer Following Pneumonectomy" Journal of Clinical Medicine 11, no. 23: 7188. https://doi.org/10.3390/jcm11237188
APA StyleQu, C., Li, R., Han, J., Yue, W., & Tian, H. (2022). Effects of Neoadjuvant Radiotherapy on Survival in Patients with Stage IIIA-N2 Non-Small-Cell Lung Cancer Following Pneumonectomy. Journal of Clinical Medicine, 11(23), 7188. https://doi.org/10.3390/jcm11237188






