Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients
Abstract
1. Introduction
2. Material and Methods
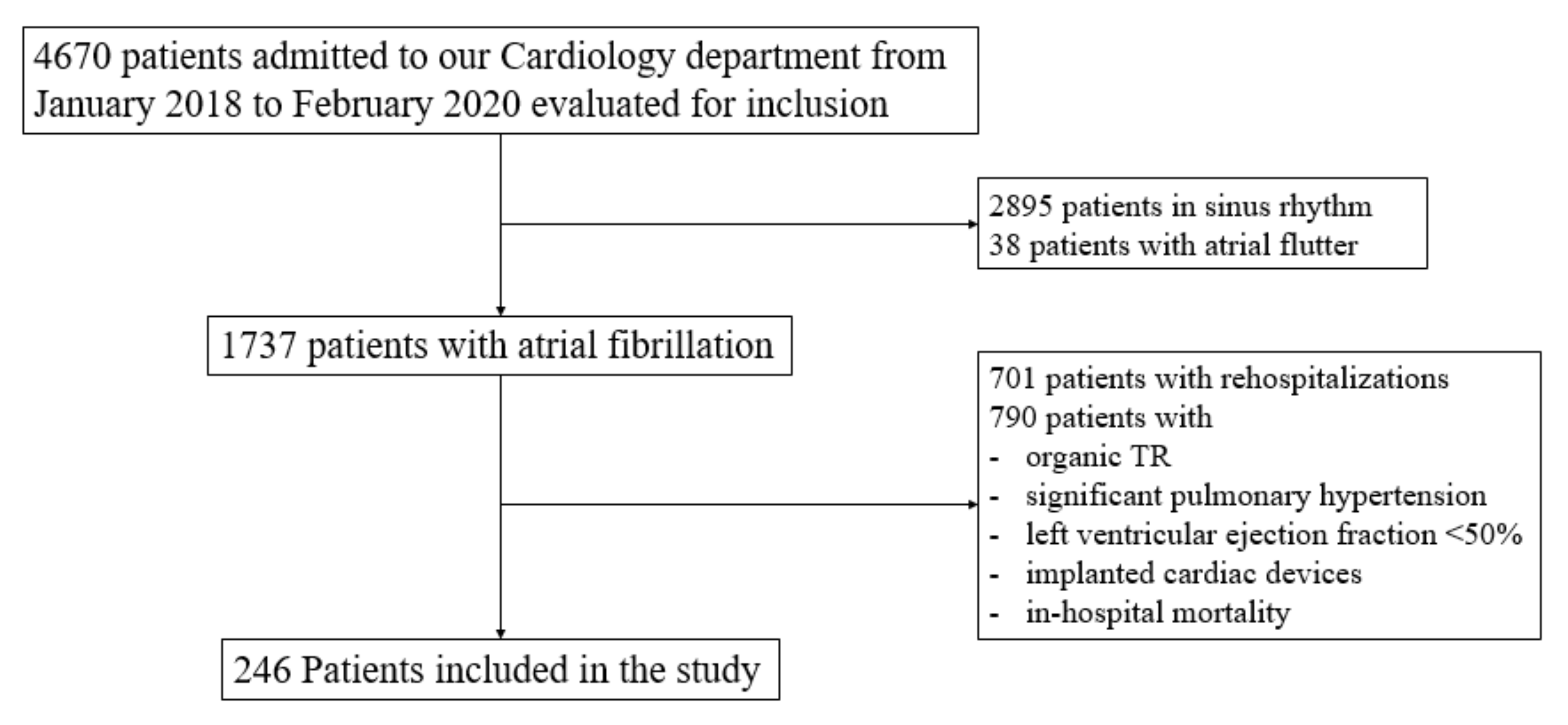
2.1. Population
2.2. Definitions
2.3. Laboratory Measurements
2.4. Statistical Analysis
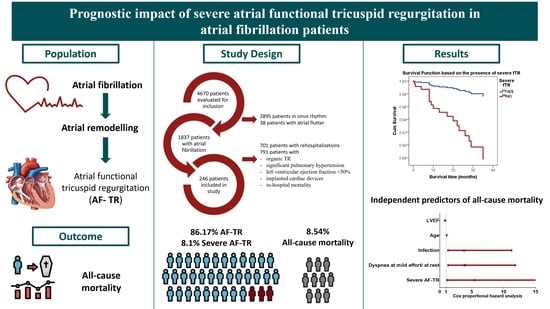
3. Results
3.1. Characteristics of Patients with Severe AF-TR
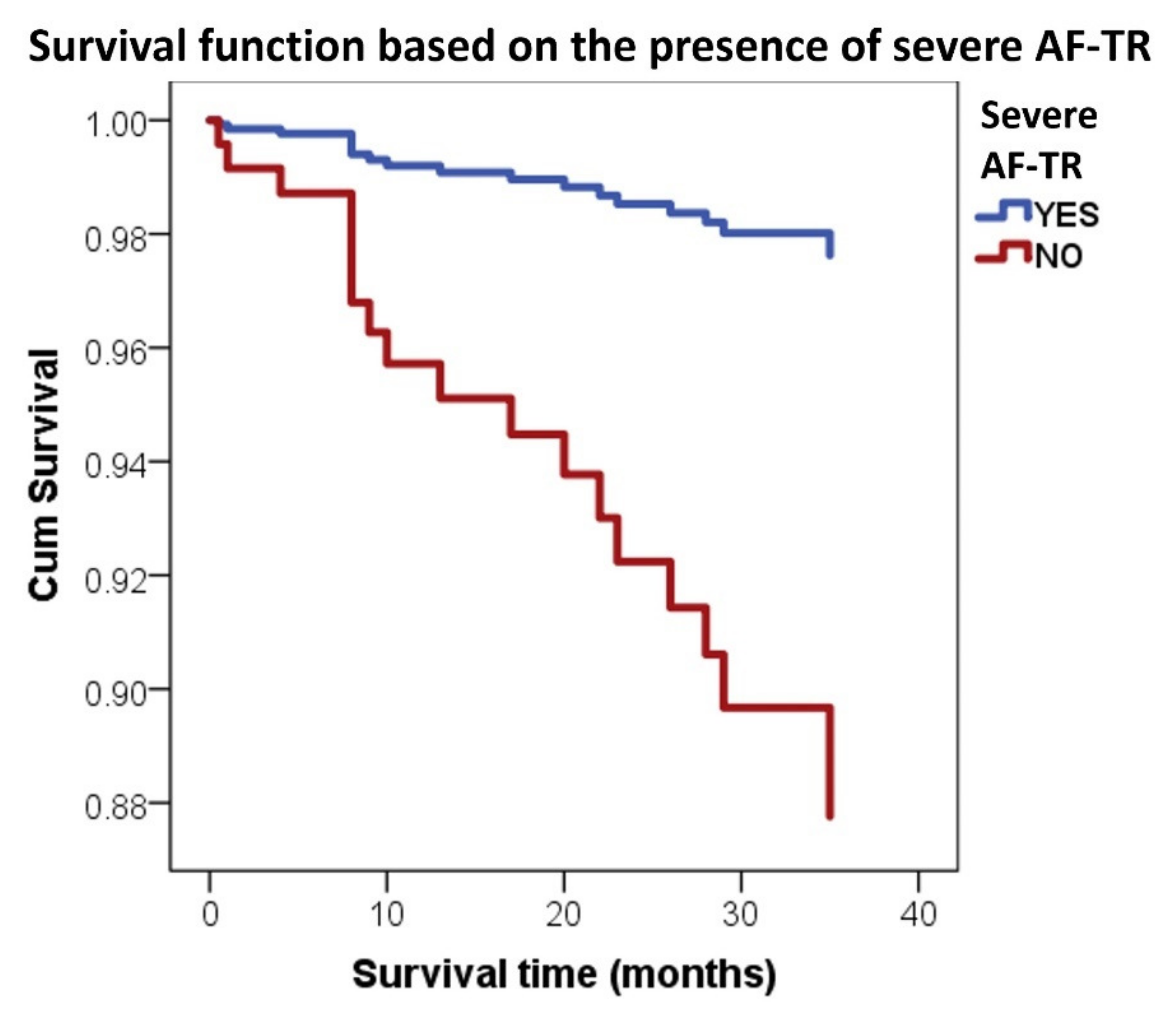
3.2. Severe AF-TR and Mortality
4. Discussion
4.1. Atrial Functional Tricuspid Regurgitation
4.2. Severe AF-TR and Mortality of AF Patients
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Vinter, N.; Huang, Q.; Fenger-Grøn, M.; Frost, L.; Benjamin, E.; Trinquart, L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): Community based cohort study. BMJ 2020, 370, m2724. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: Results from the biomarcare consortium (Biomarker for cardiovascular risk assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef]
- Staerk, L.; Wang, B.; Preis, S.R.; Larson, M.G.; Lubitz, S.A.; Ellinor, P.T.; McManus, D.D.; Ko, D.; Weng, L.-C.; Lunetta, K.L.; et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018, 361, k1453. [Google Scholar] [CrossRef]
- Vîjan, A.E.; Daha, I.C.; Delcea, C.; Dan, G.-A. Determinants of prolonged length of hospital stay of patients with atrial fibrillation. J. Clin. Med. 2021, 10, 3715. [Google Scholar] [CrossRef]
- Fauchier, L.; Villejoubert, O.; Clementy, N.; Bernard, A.; Pierre, B.; Angoulvant, D.; Ivanes, F.; Babuty, D.; Lip, G.Y.H. Causes of Death and Influencing Factors in Patients with Atrial Fibrillation. Am. J. Med. 2016, 129, 1278–1287. [Google Scholar] [CrossRef]
- Delgado, V.; Bax, J.J. Atrial Functional Mitral Regurgitation: From Mitral Annulus Dilatation to Insufficient Leaflet Remodeling. Circ. Cardiovasc. Imaging 2017, 10, 8–10. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Itabashi, Y.; Mihara, H.; Berdejo, J.; Kobayashi, S.; Siegel, R.J.; Shiota, T. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ. Cardiovasc. Imaging 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef]
- Topilsky, Y.; Khanna, A.; Le Toumeau, T.; Park, S.; Michelena, H.; Suri, R.; Mahoney, D.W.; Enriquez-Sarano, M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 314–323. [Google Scholar] [CrossRef]
- Spinner, E.M.; Shannon, P.; Buice, D.; Jimenez, J.H.; Veledar, E.; del Nido, P.J.; Adams, D.H.; Yoganathan, A.P. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 2011, 124, 920–929. [Google Scholar] [CrossRef]
- Najib, M.Q.; Vinales, K.L.; Vittala, S.S.; Challa, S.; Lee, H.R.; Chaliki, H.P. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Muraru, D.; Guta, A.C.; Ochoa-Jimenez, R.C.; Bartos, D.; Aruta, P.; Mihaila, S.; Popescu, B.A.; Iliceto, S.; Basso, C.; Badano, L.P. Functional Regurgitation of Atrioventricular Valves and Atrial Fibrillation: An Elusive Pathophysiological Link Deserving Further Attention. J. Am. Soc. Echocardiogr. 2020, 33, 42–53. [Google Scholar] [CrossRef]
- Florescu, D.R.; Muraru, D.; Volpato, V.; Gavazzoni, M.; Caravita, S.; Tomaselli, M.; Ciampi, P.; Florescu, C.; Bălșeanu, T.A.; Parati, G.; et al. Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore. J. Clin. Med. 2022, 11, 382. [Google Scholar] [CrossRef]
- Muraru, D.; Parati, G.; Badano, L.P. The tale of functional tricuspid regurgitation: When atrial fibrillation is the villain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1079–1081. [Google Scholar] [CrossRef]
- Guta, A.C.; Badano, L.P.; Tomaselli, M.; Mihalcea, D.; Bartos, D.; Parati, G.; Muraru, D. The Pathophysiological Link between Right Atrial Remodeling and Functional Tricuspid Regurgitation in Patients with Atrial Fibrillation: A Three-Dimensional Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 585–594.e1. [Google Scholar] [CrossRef]
- Matta, M.; Layoun, H.; Abou Hassan, O.K.; Rodriguez, L.; Schoenhagen, P.; Kanj, M.; Griffin, B.P.; Kapadia, S.R.; Harb, S.C. Mechanistic Insights Into Significant Atrial Functional Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 2049–2050. [Google Scholar] [CrossRef]
- Abe, Y.; Akamatsu, K.; Ito, K.; Matsumura, Y.; Shimeno, K.; Naruko, T.; Takahashi, Y.; Shibata, T.; Yoshiyama, M. Prevalence and Prognostic Significance of Functional Mitral and Tricuspid Regurgitation Despite Preserved Left Ventricular Ejection Fraction in Atrial Fibrillation Patients. Circ. J. 2018, 82, 1451–1458. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of Tricuspid Regurgitation on Long-Term Survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef]
- Topilsky, Y.; Nkomo, V.T.; Vatury, O.; Michelena, H.I.; Letourneau, T.; Suri, R.M.; Pislaru, S.; Park, S.; Mahoney, D.W.; Biner, S.; et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc. Imaging 2014, 7, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; de Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2786. [Google Scholar] [CrossRef] [PubMed]
- Prapan, N.; Ratanasit, N.; Karaketklang, K. Significant functional tricuspid regurgitation portends poor outcomes in patients with atrial fibrillation and preserved left ventricular ejection fraction. BMC Cardiovasc. Disord. 2020, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.F.; Goedemans, L.; Vo, N.M.; Prihadi, E.A.; van der Bijl, P.; Gersh, B.J.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Significant Isolated Tricuspid Regurgitation in Patients with Atrial Fibrillation Without Left-Sided Heart Disease or Pulmonary Hypertension. Am. J. Cardiol. 2020, 135, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; de Ferrari, G.M.; Knuuti, J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prognostic Implications of a Novel Algorithm to Grade Secondary Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 1085–1095. [Google Scholar] [CrossRef]
- Samaras, A.; Vrana, E.; Kartas, A.; Moysidis, D.V.; Papazoglou, A.S.; Doundoulakis, I.; Fotos, G.; Rampidis, G.; Tsalikakis, D.G.; Efthimiadis, G.; et al. Prognostic implications of valvular heart disease in patients with non-valvular atrial fibrillation. BMC Cardiovasc. Disord. 2021, 21, 453. [Google Scholar] [CrossRef]
- Muraru, D.; Caravita, S.; Guta, A.C.; Mihalcea, D.; Branzi, G.; Parati, G.; Badano, L.P. Functional Tricuspid Regurgitation and Atrial Fibrillation: Which Comes First, the Chicken or the Egg? CASE Cardiovasc. Imaging Case Rep. 2020, 4, 458. [Google Scholar] [CrossRef]
- Mutlak, D.; Lessick, J.; Reisner, S.A.; Aronson, D.; Dabbah, S.; Agmon, Y. Echocardiography-based Spectrum of Severe Tricuspid Regurgitation: The Frequency of Apparently Idiopathic Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2007, 20, 405–408. [Google Scholar] [CrossRef]
- Mutlak, D.; Khalil, J.; Lessick, J.; Kehat, I.; Agmon, Y.; Aronson, D. Risk Factors for the Development of Functional Tricuspid Regurgitation and Their Population-Attributable Fractions. JACC Cardiovasc. Imaging 2020, 13, 1643–1651. [Google Scholar] [CrossRef]
- Alonso, A.; Almuwaqqat, Z.; Chamberlain, A. Mortality in atrial fibrillation. Is it changing? Trends Cardiovasc. Med. 2021, 31, 469–473. [Google Scholar] [CrossRef]
- Itakura, K.; Hidaka, T.; Nakano, Y.; Utsunomiya, H.; Kinoshita, M.; Susawa, H.; Harada, Y.; Izumi, K.; Kihara, Y. Successful catheter ablation of persistent atrial fibrillation is associated with improvement in functional tricuspid regurgitation and right heart reverse remodeling. Heart Vessels 2020, 35, 842–851. [Google Scholar] [CrossRef]
- Masuda, M.; Sekiya, K.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; et al. Influence of catheter ablation for atrial fibrillation on atrial and ventricular functional mitral regurgitation. ESC Heart Fail. 2022, 9, 1901–1913. [Google Scholar] [CrossRef]
- He, S.; Jimenez, J.; He, Z.; Yoganathan, A.P. Mitral leaflet geometry perturbations with papillary muscle displacement and annular dilatation: An in-vitro study of ischemic mitral regurgitation. J. Heart Valve Dis. 2003, 12, 300–307. [Google Scholar]
| Total N = 246 | Non-Severe TR * N = 226 | Severe TR ** N = 20 | p Value for Comparison of * and ** | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 71.5 ± 9.4 | 71 ± 9.4 | 77 ± 7.7 | 0.005 |
| Women | 155 (63.0%) | 140 (62.0%) | 15 (75.0%) | 0.25 |
| Heart failure characteristics | ||||
| HF | 183 (74.4%) | 165 (73.0%) | 18 (90%) | 0.09 |
| ADHF | 65 (26.4%) | 55 (24.3%) | 10 (50%) | 0.01 |
| NYHA class 1–2 | 148 (60.2%) | 135 (59.7%) | 13 (65%) | 0.64 |
| NYHA class 3–4 | 35(14.2%) | 30 (13.3%) | 5 (25.0%) | 0.15 |
| AF characteristics | ||||
| Paroxysmal | 96 (39.0%) | 94 (41.6%) | 2 (10%) | 0.006 |
| Persistent | 71 (28.9%) | 66 (29.2%) | 5 (25%) | 0.7 |
| Permanent | 79 (32.1%) | 66 (29.2%) | 13 (65%) | 0.001 |
| CHA2DS2-VASc | 4 (3–5) | 4 (3–5) | 5 (4–5) | 0.04 |
| HAS-BLED | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.26 |
| Cardiovascular risk factors and comorbidities | ||||
| HTN | 215 (87.4%) | 198 (87.6%) | 17 (85%) | 0.74 |
| IHD | 66 (26.8%) | 64 (28.3%) | 2 (10%) | 0.08 |
| Prior MI | 15 (6.1%) | 14 (6.2%) | 1 (5%) | 0.83 |
| TIA/Stroke | 34 (13.8%) | 32 (14.2%) | 2 (10%) | 0.60 |
| Diabetes mellitus | 63 (25.6%) | 58 (25.7%) | 5 (25%) | 0.94 |
| Dyslipidemia | 197 (80.1%) | 186 (82.3%) | 11 (55%) | 0.003 |
| Obesity | 90 (36.6%) | 86 (38.0%) | 4 (20%) | 0.11 |
| Dementia | 9 (3.7%) | 9 (4%) | 0 (0%) | 0.36 |
| Infection | 31 (12.8%) | 28 (12.5%) | 3 (15.8%) | 0.68 |
| HR, bpm | 84.3 ± 23.1 | 84.2 ± 23.3 | 84.4 ± 20.7 | 0.75 |
| Echocardiographic characteristics | ||||
| LAD, mm | 43.5 ± 5.5 | 43.3 ± 5.5 | 46.1 ± 5.4 | 0.03 |
| LVEDD, mm | 48.1 ± 5.9 | 48.3 ± 5.8 | 45.5 ± 6 | 0.06 |
| LVESD, mm | 31.3 ± 5.4 | 31.5 ± 5.4 | 29.0 ± 5.4 | 0.06 |
| RAD, mm | 40.5 ± 6.4 | 39.8 ± 5.6 | 48.8 ± 10.2 | <0.001 |
| RVD, mm | 32.7 ± 5.7 | 32.1 ± 5.0 | 39.1 ± 8.6 | <0.001 |
| sPAP, mmHg | 28.8 ± 6.9 | 28.2 ± 6.8 | 35.7 ± 3.6 | <0.001 |
| LVEF, % | 55.9 ± 4.2 | 56.0 ± 4.3 | 54.7 ± 4.3 | 0.19 |
| Laboratory characteristics | ||||
| NT-proBNP, pg/mL | 1086 (539–1837) | 957 (486–1784) | 1686 (1300–3763) | 0.003 |
| eGFR, mL/min/1 | 75.7 (57.6–92.3) | 77.1 (59.9–93.0) | 55.34 (51.7–75.2) | 0.007 |
| HB, g/dL | 13.6 (12.4–14.6) | 13.6 (12.5–14.7) | 12.6 (11.3–14) | 0.30 |
| ADHF, acute decompensated heart failure; AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; LVEF, left ventricle ejection fraction; HB, hemoglobin; HF, Heart failure; HR, heart rate; HTN, arterial hypertension; IHD, ischemic heart disease; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; NYHA, New York Heart Association; RAD, right atrium diameter; RVD, right ventricular diameter; TIA, transient ischemic attack; TR, tricuspid regurgitation; sPAP, systolic pulmonary arterial pressure.* Patients with non-severe AF-TR; ** Patients with severe AF-TR | ||||
| Paroxysmal AF N = 96 | Persistent AF N = 71 | Permanent AF N = 79 | p for Trend | |
|---|---|---|---|---|
| Severe TR, n | 2 | 5 | 13 | 0.0006 |
| RAD, mm | 37.4 ± 4.5 | 41.2 ± 5.7 | 43.8 ± 7.3 | <0.001 |
| RVD, mm | 30.9 ± 4.7 | 33.3 ± 4.9 | 34.2 ± 6.8 | 0.0004 |
| sPAP, mmHg | 26.8 ± 7.1 | 28.9 ± 6.6 | 31.3 ± 6.3 | <0.001 |
| LVEF, % | 56.5 ± 4.4 | 56.0. ± 3.9 | 55.2 ± 4.5 | 0.13 |
| LAD, mm | 41.5 ± 5.6 | 43.7 ± 4.7 | 45.9 ± 5.2 | <0.001 |
| LVEDD, mm | 48.6 ± 6.4 | 47.2 ± 5.7 | 48.2 ± 5.2 | 0.32 |
| LVESD, mm | 31.9 ± 5.4 | 30.7 ± 5.6 | 31.1 ± 5.2 | 0.36 |
| OR (95% CI) | p Value | |
|---|---|---|
| ADHF at admission | 3.10 (1.2–7.8) | 0.01 |
| Permanent AF | 6.40 (1.5–28.3) | 0.005 |
| AUC (95% CI) | p Value | |
| Age | 0.70 (0.58–0.81) | 0.003 |
| LAD | 0.67 (0.50–0.80) | 0.015 |
| RAD | 0.81 (0.71–0.91) | <0.001 |
| RVD | 0.80 (0.70–0.90) | <0.001 |
| sPAP | 0.82 (0.75–0.90) | <0.001 |
| NT-proBNP | 0.76 (0.67–0.85) | 0.045 |
| eGFR | 0.68 (0.57–0.80) | 0.007 |
| CHA2DS2-VASc score | 0.63 (0.52–0.74) | 0.05 |
| HR (95% CI) | p Value | |
|---|---|---|
| RAD | 1.11 (1.03–1.20) | 0.005 |
| sPAP | 1.20 (1.03–1.38) | 0.015 |
| NTproBNP | 2.37 (1.10–5.10) | 0.026 |
| OR (95% CI) | p Value | |
|---|---|---|
| Severe AF-TR | 4.37 (1.41–13.57) | 0.005 |
| ADHF | 4.32 (1.72–10.83) | <0.001 |
| NYHA class III/IV | 5.74 (2.21–14.93) | <0.001 |
| TIA/Stroke | 2.81 (1.11–7.85) | 0.04 |
| Dementia | 6.08 (1.40–26.34) | 0.006 |
| Infection | 5.27 (2.02–14.21) | <0.001 |
| AUC (95% CI) | pValue | |
| Age | 0.77 (0.66–0.88) | <0.001 |
| RVD | 0.64 (0.50–0.78) | 0.05 |
| LVEF% | 0.69 (0.56–0.81) | 0.009 |
| NT-proBNP | 0.80 (0.71–0.90) | <0.001 |
| eGFR | 0.72 (0.61–0.84) | 0.01 |
| HB | 0.63 (0.50–0.76) | 0.05 |
| HR (95% CI) | p Value | |
|---|---|---|
| Severe AF-TR | 5.4 (1.1326.17) | 0.035 |
| Dyspnea at mild effort or at rest | 3.90 (1.29–11.82) | 0.016 |
| Infection | 3.79 (1.27–11.26) | 0.017 |
| Age | 1.07 (1.01–1.14) | 0.029 |
| LVEF | 0.82 (0.72–0.95) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vîjan, A.E.; Daha, I.C.; Delcea, C.; Bădilă, E.; Dan, G.-A. Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. J. Clin. Med. 2022, 11, 7145. https://doi.org/10.3390/jcm11237145
Vîjan AE, Daha IC, Delcea C, Bădilă E, Dan G-A. Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. Journal of Clinical Medicine. 2022; 11(23):7145. https://doi.org/10.3390/jcm11237145
Chicago/Turabian StyleVîjan, Ancuța Elena, Ioana Cristina Daha, Caterina Delcea, Elisabeta Bădilă, and Gheorghe-Andrei Dan. 2022. "Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients" Journal of Clinical Medicine 11, no. 23: 7145. https://doi.org/10.3390/jcm11237145
APA StyleVîjan, A. E., Daha, I. C., Delcea, C., Bădilă, E., & Dan, G.-A. (2022). Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. Journal of Clinical Medicine, 11(23), 7145. https://doi.org/10.3390/jcm11237145