Ageing and Osteoarthritis Synergically Affect Human Synoviocyte Cells: An In Vitro Study on Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
- -
- Control (CTR): the cells re-feed with synoviocyte growth medium;
- -
- IL1β: the cells cultured in synoviocyte growth medium were supplemented with 10 ng/mL IL1β (Cell Application) to replicate the inflammatory microenvironment present in OA conditions and to maintain the OA phenotype;
- -
- H2O2: the cells cultured in synoviocyte growth medium were supplemented with 30 µM H2O2 (Farmac-Zabban, Bologna, Italy) for the induction of the oxidative stress that is typical of aging;
- -
- IL1β+H2O2: the cells cultured in synoviocyte growth medium were supplemented with 10 ng/mL IL1β and 30 µM H2O2 to evaluate the combined effect of inflammatory microenvironment and oxidative stress.
2.2. Cell Viability Assay
2.3. In Vitro Microwound Healing Model
2.4. Gene Expression Analysis
2.5. Immunoenzymatic Analysis
2.6. Statistical Analysis
3. Results
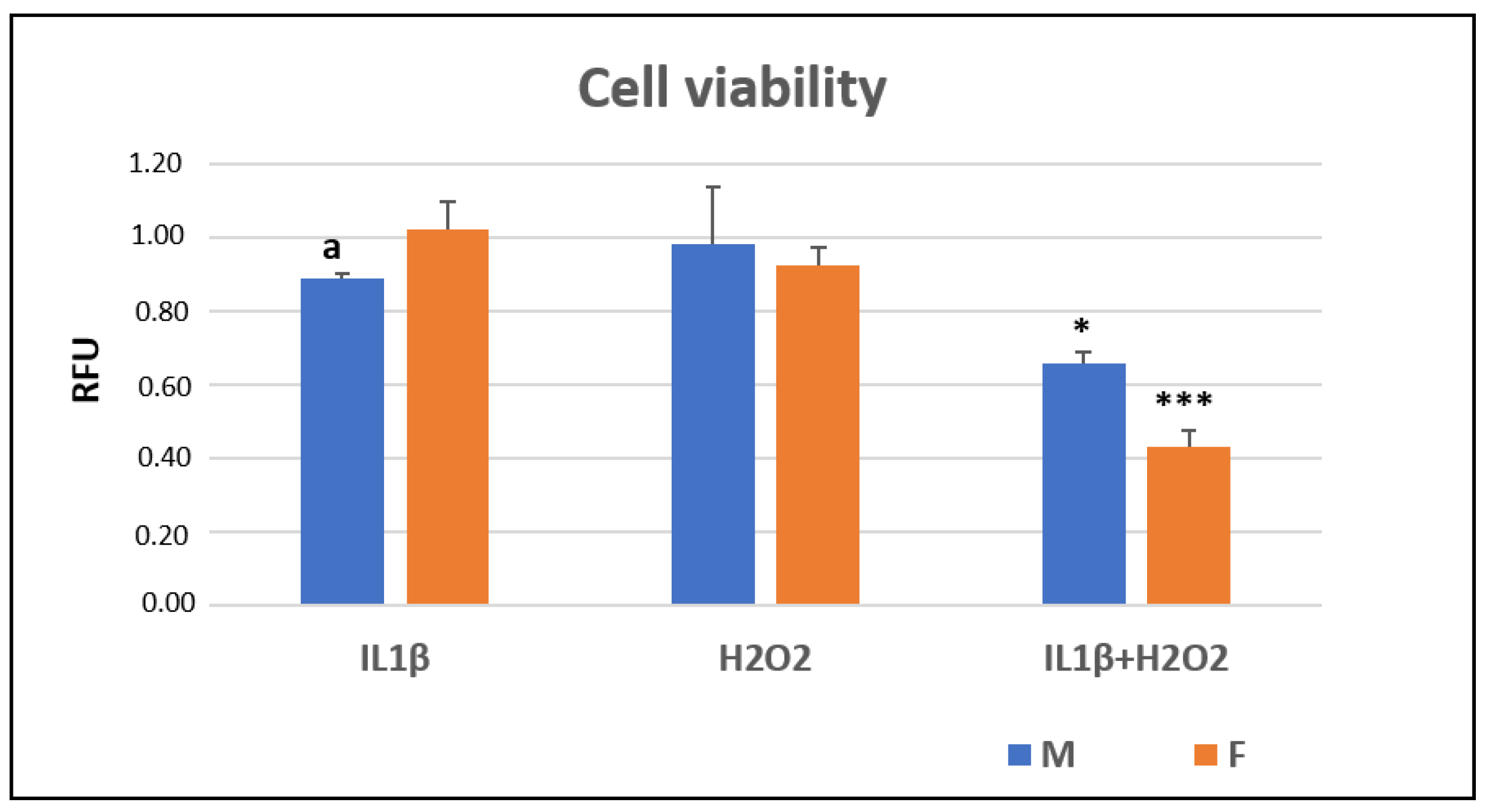
3.1. Cell Viability
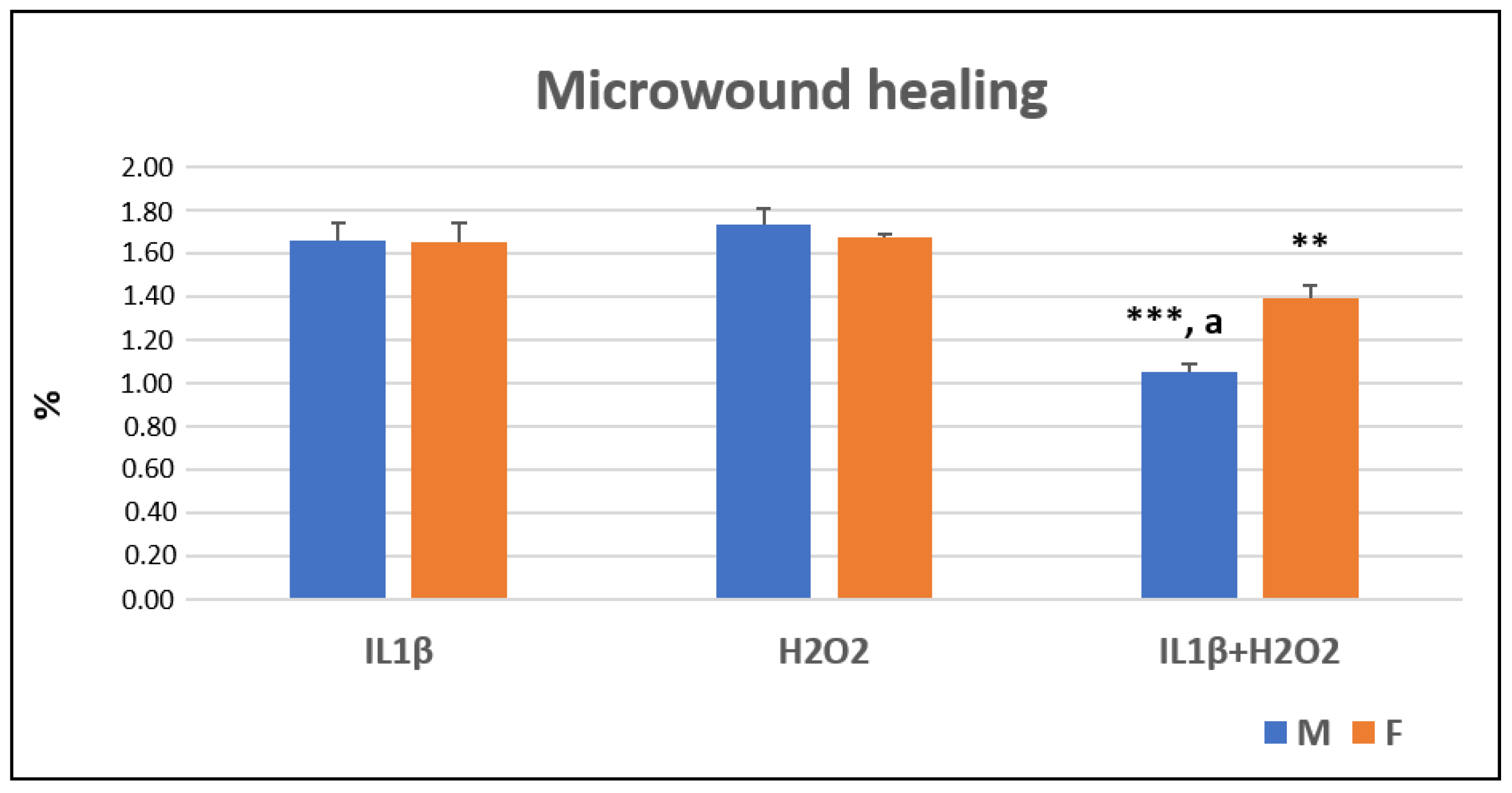
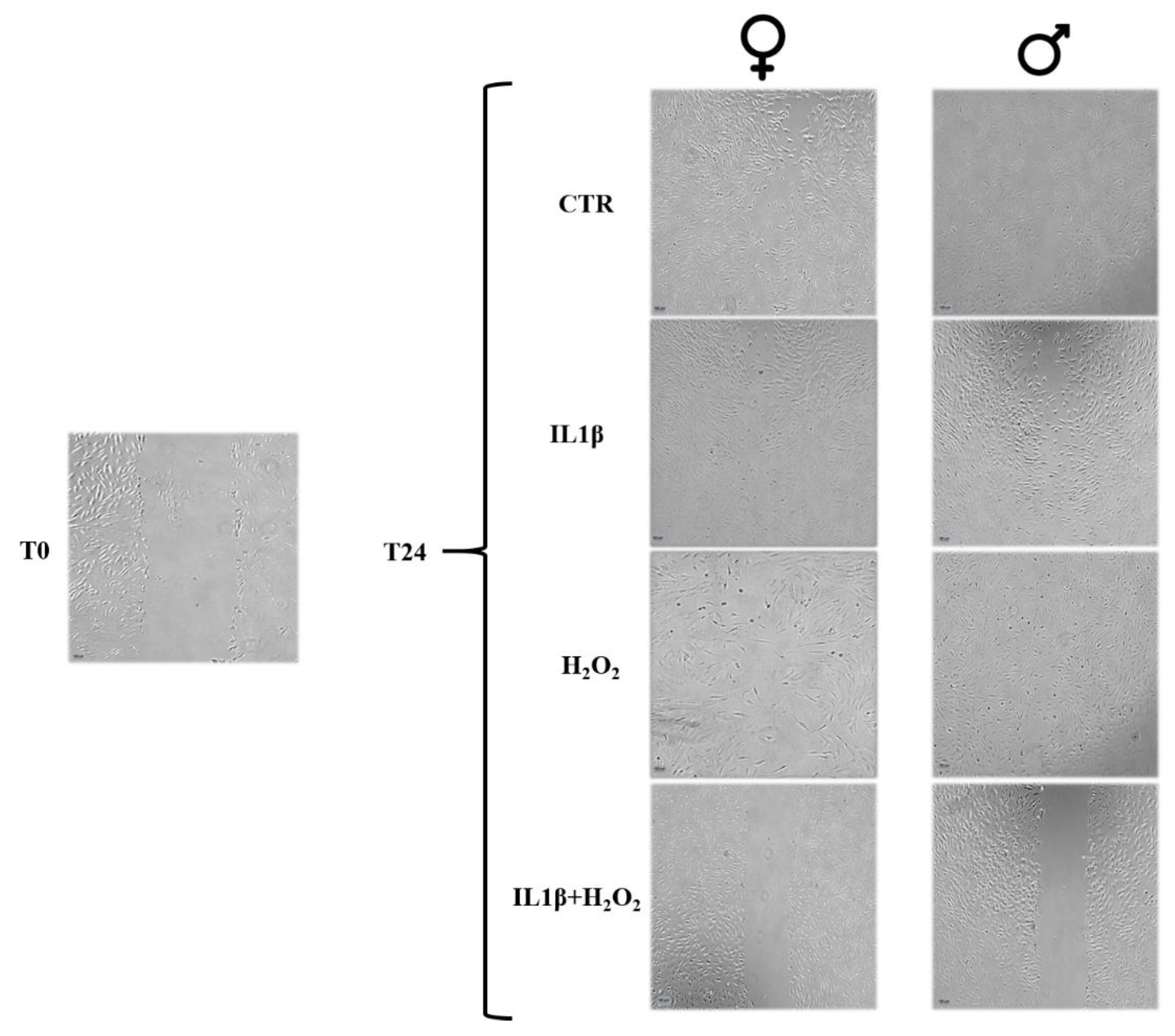
3.2. Cell Migration
3.3. Gene Expression
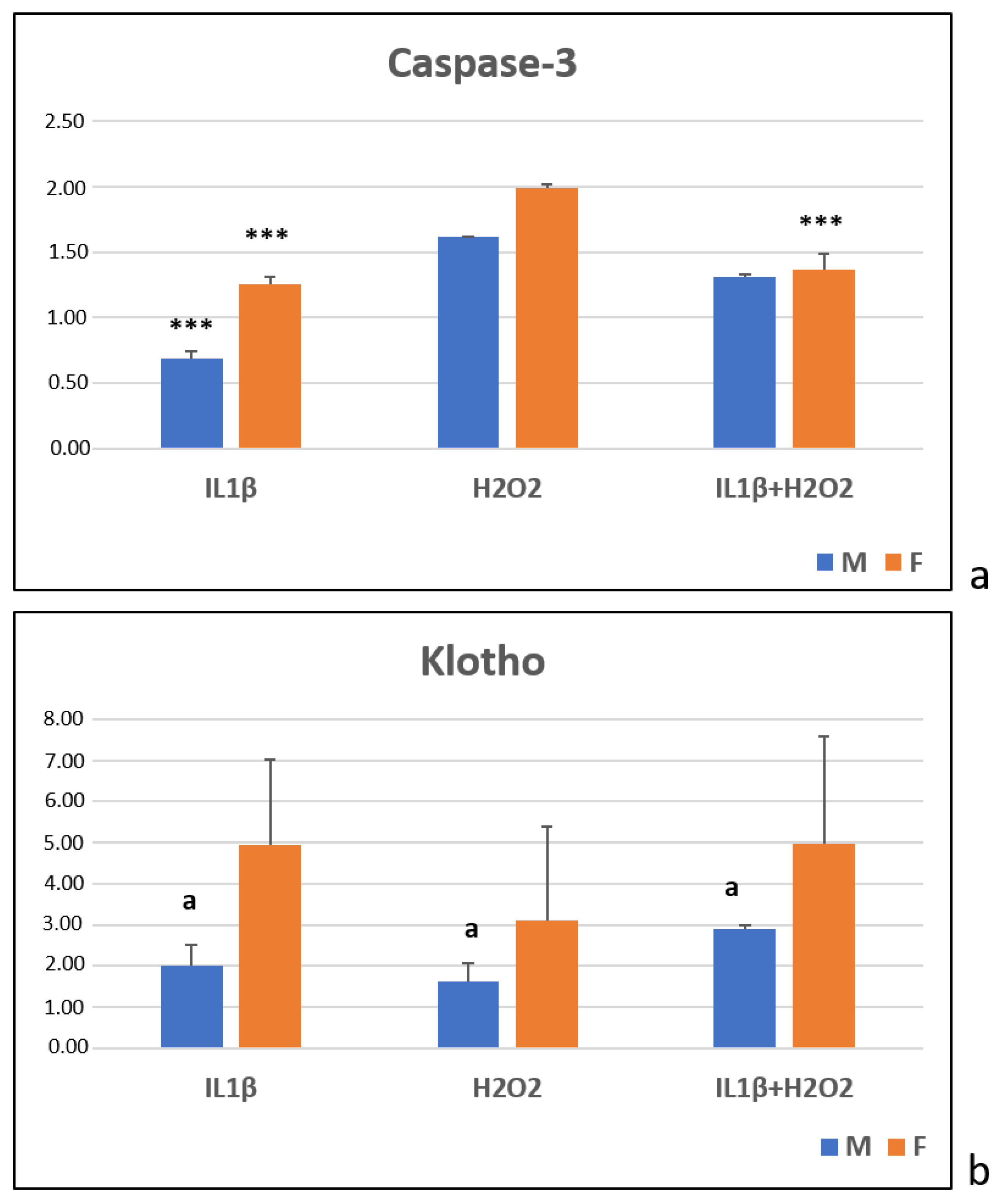
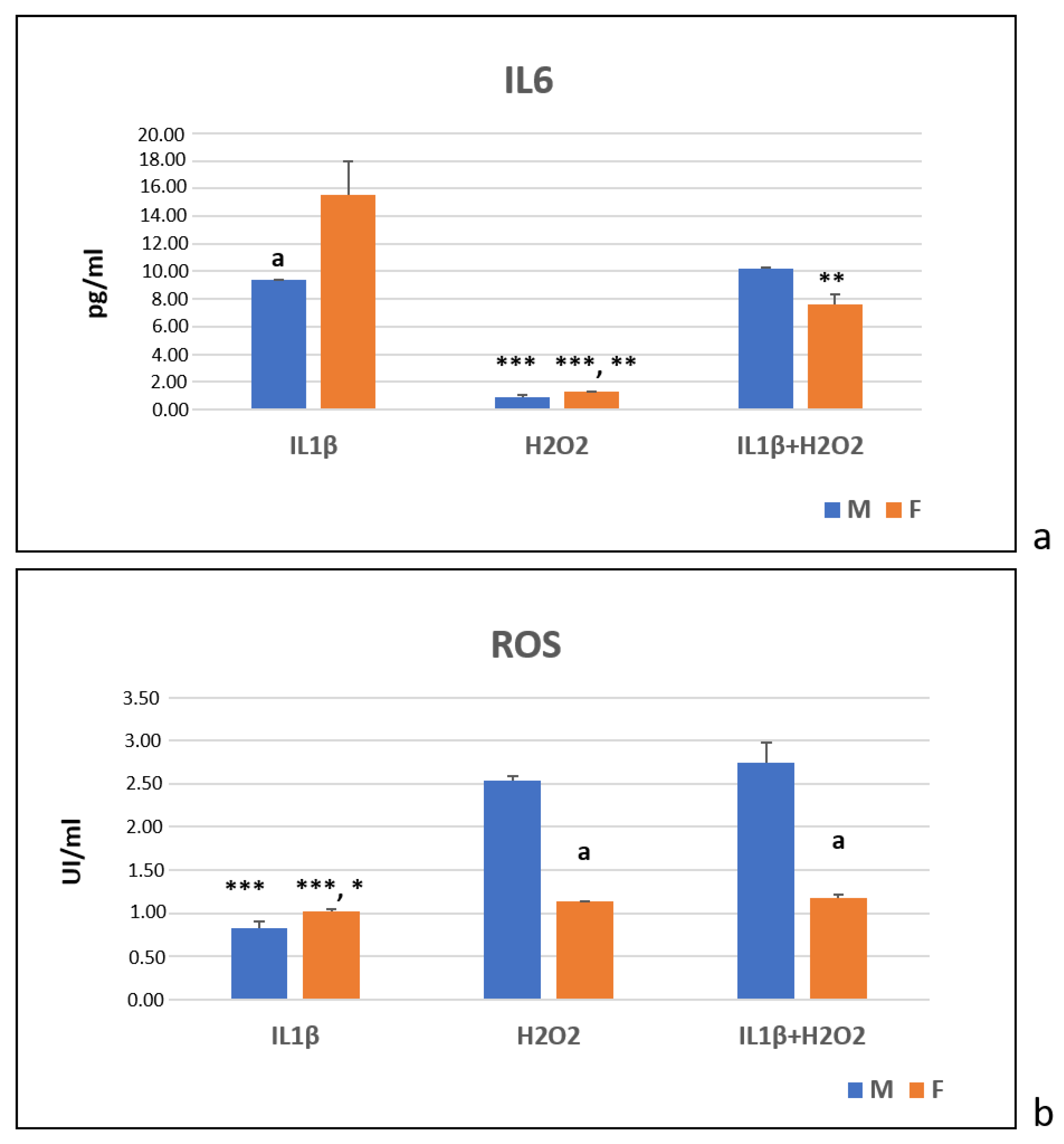
3.4. Immunoenzymatic Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef]
- Reginster, J.L.; Arden, N.K.; Haugen, I.K.; Rannou, F.; Cavalier, E.; Bruyère, O.; Branco, J.; Chapurlat, R.; Collaud Basset, S.; Al-Daghri, N.M.; et al. Guidelines for the conduct of pharmacological clinical trials in hand osteoarthritis: Consensus of a Working Group of the European Society on Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2018, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borrás, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327, Online ahead of print. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loesera, R.F. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Ye, Z.; Jang, Y.Y. Roles of reactive oxygen species in the fate of stem cells. Antioxid. Redox. Signal. 2014, 20, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Alm-Eldeen, A.; Khamis, A.; Elfiky, N.; Ahmad, R. Quercetin modulates age-induced changes in the transcript levels of some apoptosis related genes in the skeletal muscles of male rats. Braz. J. Pharm. Sci. 2020, 56, e18861. [Google Scholar] [CrossRef]
- Zharova, T.A.; Kogan, E.A.; Makarov, V.I.; Smorchkov, M.M.; Lychagin, A.V.; Ivannikov, S.V.; Zharkov, N.V.; Loschenov, V.B. Correlation of synovial caspase-3 concentration and the photodynamic effectiveness in osteoarthritis treatment. Photodiagnosis Photodyn. Ther. 2020, 30, 101669. [Google Scholar] [CrossRef]
- Veronesi, F.; Borsari, V.; Cherubini, A.; Fini, M. Association of Klotho with physical performance and frailty in middle-aged and older adults: A systematic review. Exp. Gerontol. 2021, 154, 111518. [Google Scholar] [CrossRef]
- Gu, Y.; Ren, K.; Wang, L.; Yao, Q. Loss of Klotho contributes to cartilage damage by derepression of canonical Wnt/β-catenin signaling in osteoarthritis mice. Aging 2019, 11, 12793–12809. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Yao, Y.; He, G.Y.; Wu, X.J.; Wang, C.P.; Luo, X.B.; Zhao, Y.; Long, Y. Association between environmental exposure to perchlorate, nitrate, and thiocyanate and serum α-Klotho levels among adults from the National Health and nutrition examination survey (2007–2014). BMC Geriatr. 2022, 22, 740. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Xu, Y.S.; Wang, M.M.; Chen, D.; Jiang, X.; Xiong, Z.F. Inflammatory biomarkers in older adults with frailty: A systematic review and meta-analysis of cross-sectional studies. Aging Clin. Exp. Res. 2022, 34, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chan, D.C.; Chiang, C.K.; Wang, C.C.; Yang, T.H.; Lan, K.C.; Chao, S.C.; Tsai, K.S.; Yang, R.S.; Liu, S.H. Advanced Glycation End-Products Induced VEGF Production and Inflammatory Responses in Human Synoviocytes Via RAGE-NF-kB Pathway Activation. J. Orthop. Res. 2016, 34, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Shimamura, S.; Yasuda, S.; Kono, M.; Kono, M.; Fujieda, Y.; Kato, M.; Oku, K.; Bohgaki, T.; Shimizu, T.; et al. Ectopic RASGRP2 (CalDAG-GEFI) expression in rheumatoid synovium contributes to the development of destructive arthritis. Ann. Rheum. Dis. 2018, 77, 1765–1772. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Z.; Hou, J.; Huang, T.; Yang, M. Osthole improves collagen-induced arthritis in a rat model through inhibiting inflammation and cellular stress. Cell. Mol. Biol. Lett. 2018, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like synoviocytes: Role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Contartese, D.; Tschon, M.; De Mattei, M.; Fini, M. Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 3696. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Della Bella, E.; Torricelli, P.; Pagani, S.; Fini, M. Effect of adipose-derived mesenchymal stromal cells on tendon healing in aging and estrogen deficiency: An in vitro co-culture model. Cytotherapy 2015, 17, 1536–1544. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Cellular Senescence: Molecular Mechanisms, In Vivo Significance, and Redox Considerations. Antioxid. Redox. Signal. 2009, 11, 59–98. [Google Scholar] [CrossRef]
- Xu, B.; Wang, G.; Zhang, J.; Cao, W.; Chen, X. Resveratrol decreases FoXO protein expression through PI3K-Akt-dependent pathway inhibition in H2O2-treated synoviocytes. Histol. Histopathol. 2017, 32, 1305–1315. [Google Scholar] [PubMed]
- Cha, H.S.; Bae, E.K.; Ahn, J.K.; Lee, J.; Ahn, K.S.; Koh, E.M. Slug suppression induces apoptosis via Puma transactivation in rheumatoid arthritis fibroblast-like synoviocytes treated with hydrogen peroxide. Exp. Mol. Med. 2010, 42, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lemarechal, H.; Anract, P.; Beaudeux, J.L.; Bonnefont-Rousselot, D.; Ekindjian, O.G.; Borderie, D. Impairment of thioredoxin reductase activity by oxidative stress in human rheumatoid synoviocytes. Free Radic. Res. 2007, 41, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, J.; Wang, H. MiR-30a-3p ameliorates oxidative stress in rheumatoid arthritis synovial fibroblasts via activation of Nrf2-ARE signaling pathway. Immunol. Lett. 2021, 232, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, G.; Zhang, Y.; Zhang, J.; Cao, W.; Chen, X. Effect of lentivirus-mediated overexpression or silencing of MnSOD on apoptosis of resveratrol-treated fibroblast-like synoviocytes in rheumatoid arthritis. Eur. J. Pharmacol. 2019, 844, 65–72. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Shen, S.; Zhang, L.; Xiang, Y.; Weng, X. Mst1 promotes mitochondrial dysfunction and apoptosis in oxidative stress-induced rheumatoid arthritis synoviocytes. Aging 2020, 12, 16211–16223. [Google Scholar] [CrossRef]
- Forte, L.; Sarda, S.; Torricelli, P.; Combes, C.; Brouillet, F.; Marsan, O.; Salamanna, F.; Fini, M.; Boanini, E.; Bigi, A. Multifunctionalization Modulates Hydroxyapatite Surface Interaction with Bisphosphonate: Antiosteoporotic and Antioxidative Stress Materials. ACS Biomater. Sci. Eng. 2019, 5, 3429–3439. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Rubini, K.; Fini, M.; Bigi, A. Quercetin and alendronate multi-functionalized materials as tools to hinder oxidative stress damage. J. Biomed. Mater. Res. A 2017, 105, 3293–3303. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Pan, J.; Yang, W.; Zeng, L.; Liu, J. Efficacy of Duhuo Jisheng Decoction for Treating Cold-Dampness Obstruction Syndrome-Type Knee Osteoarthritis: A Pooled Analysis. Biomed. Res. Int. 2022, 2022, 2350404. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Minguzzi, M.; Sicuro, L.; Veronesi, F.; Santi, S.; Scotto D’Abusco, A.; Fini, M.; Borzì, R.M. The N-Acetyl Phenylalanine Glucosamine Derivative Attenuates the Inflammatory/Catabolic Environment in a Chondrocyte-Synoviocyte Co-Culture System. Sci. Rep. 2019, 9, 13603. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Borsari, V.; Veronesi, F.; Ferrari, A.; Cepollaro, S.; Torricelli, P.; Filardo, G.; Fini, M. Increased Chondrogenic Potential of Mesenchymal Cells From Adipose Tissue Versus Bone Marrow-Derived Cells in Osteoarthritic In Vitro Models. J. Cell. Physiol. 2017, 232, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Blasioli, D.J.; Kaplan, D.L. The roles of catabolic factors in the development ofosteoarthritis. Tissue Eng. Part B Rev. 2014, 20, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281–286. [Google Scholar] [CrossRef]
- Dai, S.M.; Shan, Z.Z.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K.; Yudoh, K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: Possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006, 54, 818–831. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, L.; Li, P.; Wu, Y.; Zhang, P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol. Med. Rep. 2015, 12, 7086–7092. [Google Scholar] [CrossRef]
- Collins, J.A.; Wood, S.T.; Nelson, K.J.; Rowe, M.A.; Carlson, C.S.; Chubinskaya, S.; Poole, L.B.; Furdui, C.M.; Loeser, R.F. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J. Biol. Chem. 2016, 291, 6641–6654. [Google Scholar] [CrossRef]
- Altindag, O.; Erel, O.; Aksoy, N.; Selek, S.; Celik, H.; Karaoglanoglu, M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol. Int. 2007, 27, 339–344. [Google Scholar] [CrossRef]
- Carlo, M.D., Jr.; Loeser, R.F. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003, 48, 3419–3430. [Google Scholar] [CrossRef]
- Xue, X.T.; Zhang, T.; Cui, S.J.; He, D.Q.; Wang, X.D.; Yang, R.L.; Liu, D.W.; Liu, Y.; Gan, Y.H.; Kou, X.X.; et al. Sexual dimorphism of estrogen-sensitized synoviocytes contributes to gender difference in temporomandibular joint osteoarthritis. Oral Dis. 2018, 24, 1503–1513. [Google Scholar] [CrossRef]
- Li, C.J.; Xiao, Y.; Sun, Y.C.; He, W.Z.; Liu, L.; Huang, M.; He, C.; Huang, M.; Chen, K.X.; Hou, J.; et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. 2021, 33, 1957–1973. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 2021, 10, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, Z.; Guo, Y.; Qi, X.; Yang, Y.; Jia, X.; Li, R.; Shi, J.; Gao, W.; Ren, Z.; et al. Bioinspired Nanovesicles Convert the Skeletal Endothelium-Associated Secretory Phenotype to Treat Osteoporosis. ACS Nano 2022, 16, 11076–11091. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar]
- De Luca, A.; Raimondi, L.; Salamanna, F.; Carina, V.; Costa, V.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Relevance of 3d culture systems to study osteosarcoma environment. J. Exp. Clin. Cancer Res. 2018, 37, 2. [Google Scholar] [CrossRef]
- Salamanna, F.; Borsari, V.; Brogini, S.; Torricelli, P.; Cepollaro, S.; Cadossi, M.; Fini, M. A Human 3D In Vitro Model to Assess the Relationship Between Osteoporosis and Dissemination to Bone of Breast Cancer Tumor Cells. J. Cell. Physiol. 2017, 232, 1826–1834. [Google Scholar] [CrossRef]
- Pagani, S.; Torricelli, P.; Veronesi, F.; Salamanna, F.; Cepollaro, S.; Fini, M. An advanced tri-culture model to evaluate the dynamic interplay among osteoblasts, osteoclasts, and endothelial cells. J. Cell. Physiol. 2018, 233, 291–301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Contartese, D.; Borsari, V.; Pagani, S.; Fini, M.; De Mattei, M.; Tschon, M. Ageing and Osteoarthritis Synergically Affect Human Synoviocyte Cells: An In Vitro Study on Sex Differences. J. Clin. Med. 2022, 11, 7125. https://doi.org/10.3390/jcm11237125
Veronesi F, Contartese D, Borsari V, Pagani S, Fini M, De Mattei M, Tschon M. Ageing and Osteoarthritis Synergically Affect Human Synoviocyte Cells: An In Vitro Study on Sex Differences. Journal of Clinical Medicine. 2022; 11(23):7125. https://doi.org/10.3390/jcm11237125
Chicago/Turabian StyleVeronesi, Francesca, Deyanira Contartese, Veronica Borsari, Stefania Pagani, Milena Fini, Monica De Mattei, and Matilde Tschon. 2022. "Ageing and Osteoarthritis Synergically Affect Human Synoviocyte Cells: An In Vitro Study on Sex Differences" Journal of Clinical Medicine 11, no. 23: 7125. https://doi.org/10.3390/jcm11237125
APA StyleVeronesi, F., Contartese, D., Borsari, V., Pagani, S., Fini, M., De Mattei, M., & Tschon, M. (2022). Ageing and Osteoarthritis Synergically Affect Human Synoviocyte Cells: An In Vitro Study on Sex Differences. Journal of Clinical Medicine, 11(23), 7125. https://doi.org/10.3390/jcm11237125








