Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study
Abstract
1. Background
2. Methods
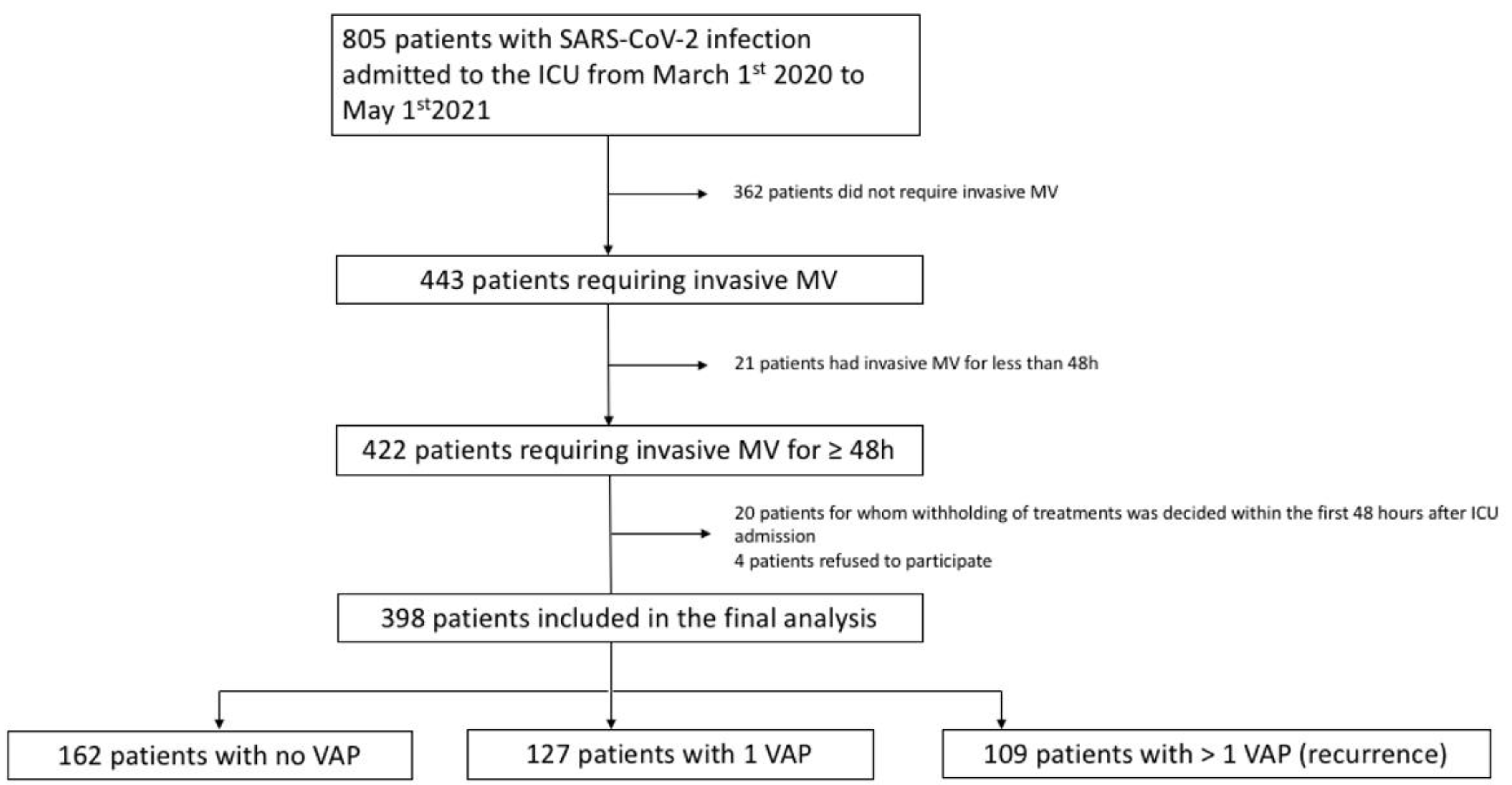
2.1. Study Design and Population
2.2. Definitions
VAP Diagnosis
- -
- New or progressive persistent infiltration on chest radiograph;
- -
- At least two of the following: new onset of fever, purulent endotracheal aspirate, leukocytosis or leucopenia, increased minute ventilation, arterial oxygenation decline, need for increased vasopressor infusion to maintain blood pressure (for patients with ARDS, for whom demonstration of radiologic deterioration is difficult, at least two of the preceding criteria sufficed);
- -
- A positive quantitative or qualitative culture from broncho-alveolar lavage (BAL), protected distal sample (PDS) or endotracheal aspirate (ETA).
2.3. Bacterial Co-Infection at ICU Admission
2.4. Relapse and Recurrence of VAP
2.5. Baseline Assessment and Data Collection
- Dexamethasone (at 6 or 12 mg per day) [15]
- Methylprednisolone for persistent ARDS as described elsewhere [16]
- Hydrocortisone (at 200 mg per day)
- Interleukine-6 (Il-6) receptor antagonist (Tocilizumab) [17]
- Interleukin-1(II-1) receptor antagonist (Anakinra) [18]
- Janus Kinases (JAK) receptor antagonist (Ruxolitinib) [18]
- The combination of several of them during the same ICU stay
2.6. Antibiotic Treatment
2.7. Management of Antibiotic Treatment
2.8. Study Outcomes
2.9. Statistical Analysis
3. Results
3.1. Patients’ Characteristics at ICU Admission
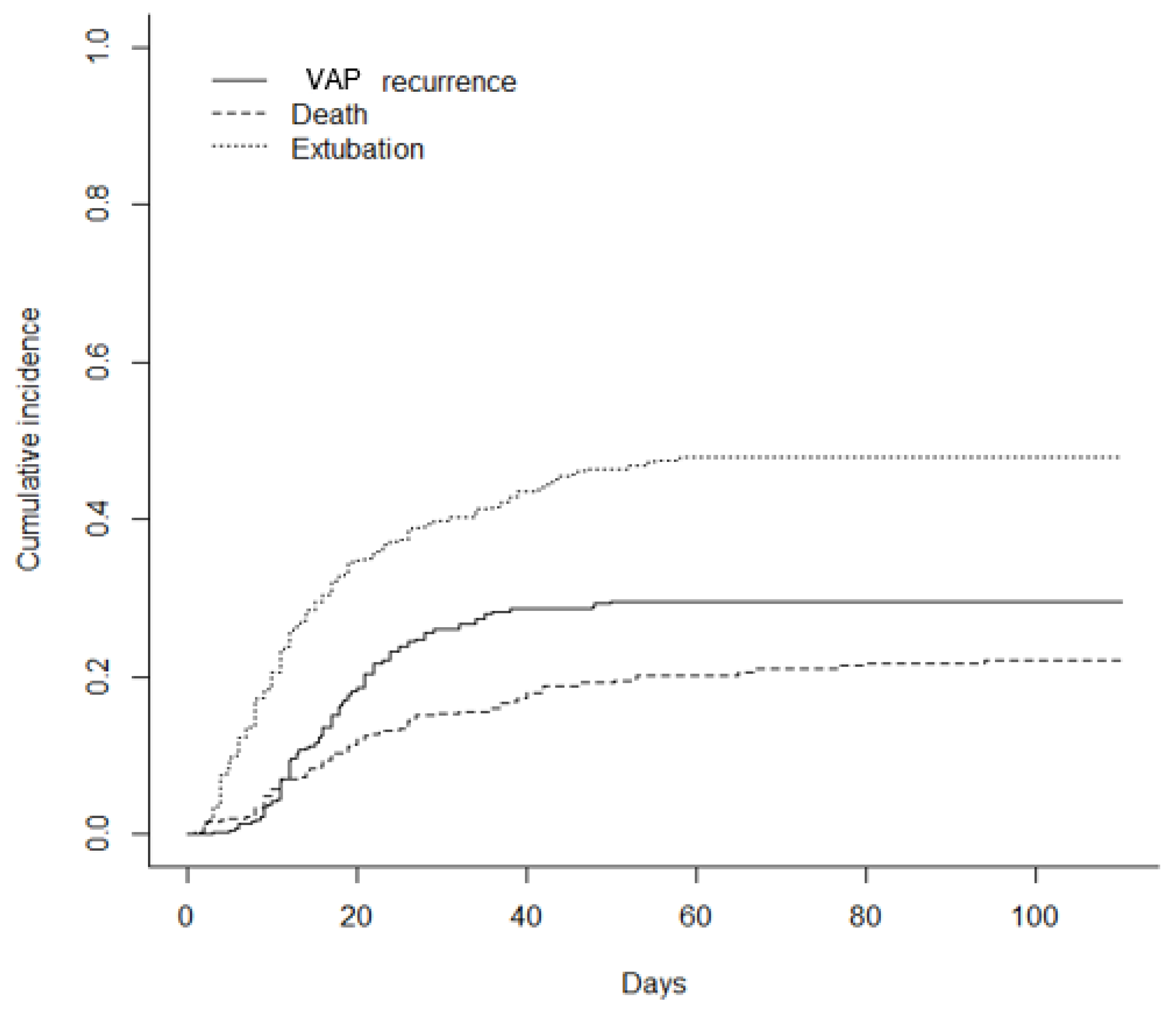
3.2. VAP and Recurrence Incidence
3.3. Use of Immunosuppressive Therapies during the ICU Stays
3.4. Microbiological and Pharmacological Results
3.5. Clinical Outcomes
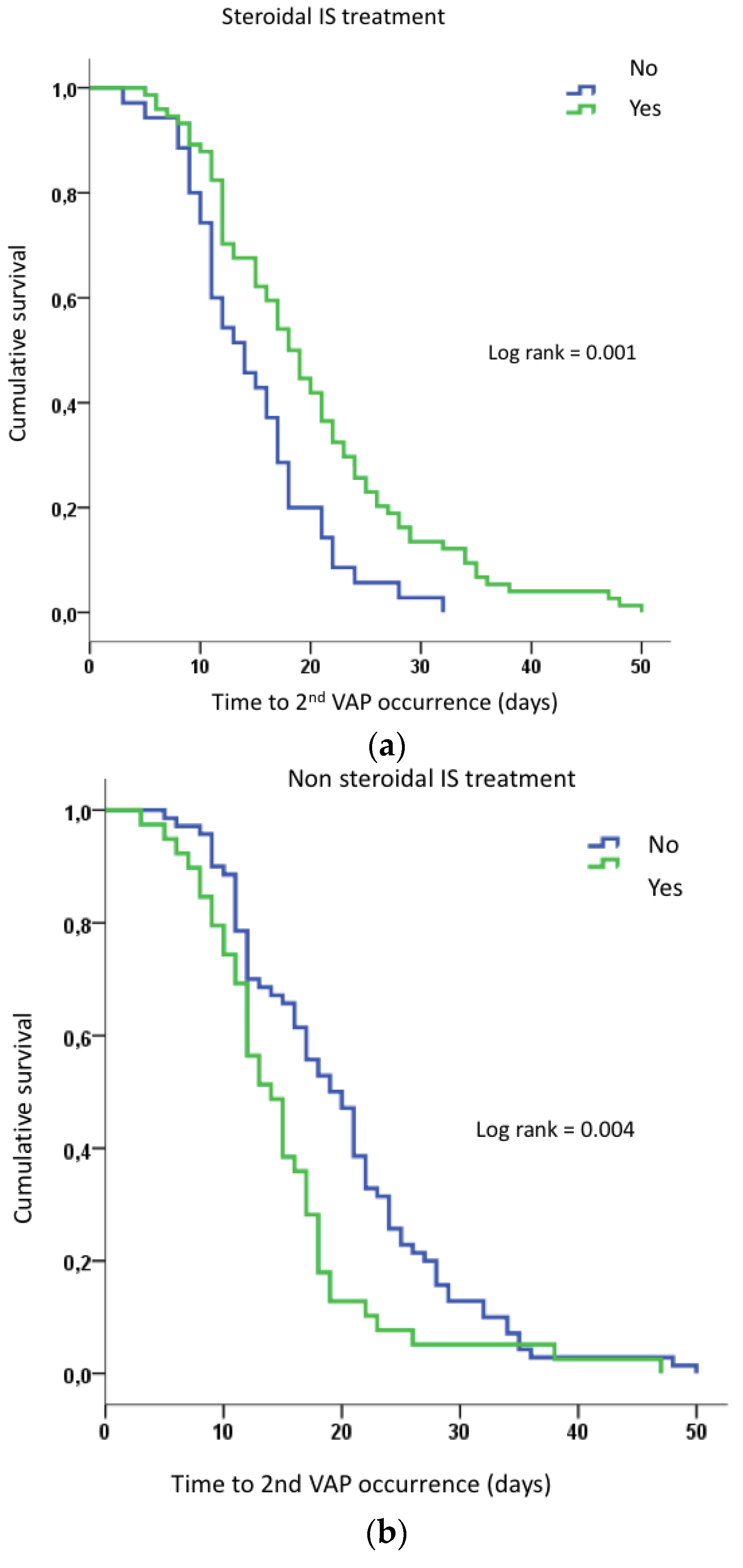
3.6. Factors Associated with VAP Recurrences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ARDS | acute respiratory distress syndrome |
| BAL | broncho-alveolar lavage |
| ECMO | extracorporeal membrane oxygenation |
| ETA | endotracheal aspirate |
| IL-1 | interleukine 1 |
| IL-6 | interleukine 6 |
| ICU | intensive care unit |
| IS | immunosuppressive |
| JAK | janus kinase |
| MSSA | methicillin-susceptible Staphylococcus aureus |
| MV | mechanical ventilation |
| PSD | protected distal sample |
| PCR | polymerase chain reaction |
| RT-PCR | reverse transcription polymerase chain reaction |
| SAPS II | simplified acute physiologic Score II |
| SOFA | sequential organ failure assessment |
| SRLF | société de réanimation de langue française |
| VAP | ventilator-associated pneumonia |
| VFD | ventilator free days |
References
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Gragueb-Chatti, I.; Lopez, A.; Hamidi, D.; Guervilly, C.; Loundou, A.; Daviet, F.; Cassir, N.; Papazian, L.; Forel, J.-M.; Leone, M.; et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: A multicenter retrospective study. Ann. Intensive Care 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W.; Joshi, I.; Leal, L.; Ooi, E.E. Immune Immunomodulation in Coronavirus Disease 2019 (COVID-19): Strategic Considerations for Personalized Therapeutic Intervention. Clin. Infect. Dis. 2022, 74, 144–148. [Google Scholar] [CrossRef]
- Cour, M.; Simon, M.; Argaud, L.; Monneret, G.; Venet, F. Effects of dexamethasone on immune dysfunction and ventilator-associated pneumonia in COVID-19 acute respiratory distress syndrome: An observational study. J. Intensive Care 2021, 9, 64. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA 2020, 324, 1330. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Sahnoun, T.; Gautier, M.; Vidal, P.; Burrel, S.; Pineton de Chambrun, M.; Chommeloux, J.; Desnos, C.; Arzoine, J.; Nieszkowska, A.; et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann. Intensive Care 2020, 10, 158. [Google Scholar] [CrossRef]
- Gregorova, M.; Morse, D.; Brignoli, T.; Steventon, J.; Hamilton, F.; Albur, M.; Arnold, D.; Thomas, M.; Halliday, A.; Baum, H.; et al. Post-acute COVID-19 associated with evidence of bystander T-cell activation and a recurring antibiotic-resistant bacterial pneumonia. eLife 2020, 9, e63430. [Google Scholar] [CrossRef]
- Fumagalli, J.; Panigada, M.; Klompas, M.; Berra, L. Ventilator-associated pneumonia among SARS-CoV-2 acute respiratory distress syndrome patients. Curr. Opin. Crit. Care 2022, 28, 74–82. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- François, B.; Laterre, P.-F.; Luyt, C.-E.; Chastre, J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit. Care 2020, 24, 289. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone Infusion in Early Severe ARDS. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association between Early Treatment with Tocilizumab and Mortality among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021, 181, 41. [Google Scholar] [CrossRef]
- Kaplanski, G.; Bontemps, D.; Esnault, P.; Blasco, V.; Carvelli, J.; Delarbre, D.; Cauchois, R.; Forel, J.-M.; Papazian, L. Combined Anakinra and Ruxolitinib treatment to rescue extremely ill COVID-19 patients: A pilot study. Autoimmun. Rev. 2021, 20, 102726. [Google Scholar] [CrossRef]
- Martin, C.; Brun-Buisson, C. Prise en charge initiale des états septiques graves de l’adulte et de l’enfant. Réanimation 2007, 16, S1–S21. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Antibiothérapie des infections à entérobactéries et à Pseudomonas aeruginosa chez l’adulte: Place des carbapénèmes et de leurs alternatives. Recomm. Bonne Prat. 2019. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2019-06/synthese_infections_enterobacteries.pdf (accessed on 9 October 2022).
- Bouadma, L.; Lescure, F.-X.; Lucet, J.-C.; Yazdanpanah, Y.; Timsit, J.-F. Severe SARS-CoV-2 infections: Practical considerations and management strategy for intensivists. Intensive Care Med. 2020, 46, 579–582. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.-R.; Paiva, J.-A.; Timsit, J.-F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Razazi, K.; Arrestier, R.; Haudebourg, A.F.; Benelli, B.; Carteaux, G.; Decousser, J.; Fourati, S.; Woerther, P.L.; Schlemmer, F.; Charles-Nelson, A.; et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit. Care 2020, 24, 699. [Google Scholar] [CrossRef]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lemeur, A.; Planche, L.; Lascarrou, J.-B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25, 72. [Google Scholar] [CrossRef]
- Llitjos, J.-F.; Bredin, S.; Lascarrou, J.-B.; Soumagne, T.; Cojocaru, M.; Leclerc, M.; Lepetit, A.; Gouhier, A.; Charpentier, J.; Piton, G.; et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: A multicentre retrospective cohort study. Ann. Intensive Care 2021, 11, 20. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between Administration of IL-6 Antagonists and Mortality among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499. [Google Scholar] [CrossRef]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Beaucoté, V.; Plantefève, G.; Tirolien, J.-A.; Desaint, P.; Fraissé, M.; Contou, D. Lung Abscess in Critically Ill Coronavirus Disease 2019 Patients with Ventilator-Associated Pneumonia: A French Monocenter Retrospective Study. Crit. Care Explor. 2021, 3, e0482. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults: A Randomized Trial. JAMA 2003, 290, 2588. [Google Scholar] [CrossRef]
- Chastre, J.; Luyt, C.E. Optimising the duration of antibiotic therapy for ventilator-associated pneumonia. Eur. Respir. Rev. 2007, 16, 40–44. [Google Scholar] [CrossRef]
- Trouillet, J. Les Pneumopathies Acquises Sous Ventilation Mécanique. 2009. Available online: https://sfar.org/les-pneumopathies-acquises-sous-ventilation-mecanique/ (accessed on 9 October 2022).
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Boumaza, A.; Gay, L.; Mezouar, S.; Bestion, E.; Diallo, A.B.; Michel, M.; Desnues, B.; Raoult, D.; La Scola, B.; Halfon, P.; et al. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. J. Infect. Dis. 2021, 224, 395–406. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef]
- Nseir, S.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Makris, D.; Boulle Geronimi, C.; et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the coVAPid cohort. Crit. Care 2021, 25, 177. [Google Scholar] [CrossRef]
| TOTAL (n = 398) | 0 VAP (n = 162) | 1 VAP (n = 127) | >1 VAP (n = 109) | p | |
|---|---|---|---|---|---|
| Age, years ± SD | 65 ± 12 | 63 ± 12 | 66 ± 12 | 66 ± 10 | 0.445 |
| Male, n (%) | 287 (72.1) | 112 (69.1) | 93 (73.2) | 82 (75.2) | 0.517 |
| SAPS II, mean ± SD | 40 (31–51) | 40 (31–45) | 40 (33–48) | 42(34–51) | 0.369 |
| SOFA, mean ± SD | 5 (3–8) | 5 (3–7) | 5 (3–8) | 6 (4–8) | 0.479 |
| COMORBIDITIES, n (%) | |||||
| Chronic Heart Failure | 71 (17.8) | 28 (17.3) | 27 (21.3) | 16 (14.7) | 0.408 |
| Chronic respiratory failure | 48 (12.1) | 20 (12.3) | 16 (12.6) | 12 (11) | 0.923 |
| Chronic kidney failure | 29 (7.3) | 9 (5.6) | 12 (9.4) | 8 (7.3) | 0.450 |
| Hypertension | 193 (48.5) | 80 (49.4) | 58 (45.7) | 55 (50.5) | 0.731 |
| Diabetes mellitus | 140 (35.2) | 51 (31.5) | 45 (35.4) | 44 (40.4) | 0.323 |
| Smoker | 93 (23.4) | 45 (27.8) | 22 (17.3) | 26 (23.9) | 0.114 |
| Obesity | 161 (40.5) | 72 (44.4) | 43 (33.9) | 46 (42.2) | 0.174 |
| History of neoplasm | 42 (10.6) | 15 (9.3) | 15 (11.8) | 12 (11) | 0.766 |
| Immunosuppression | 39 (9.8) | 15 (9.3) | 11 (8.7) | 13 (11.9) | 0.671 |
| Admission periods, n (%) | |||||
| First wave | 67 (16.8) | 27 (16.7) | 23 (18.1) | 17 (15.6) | 0.874 |
| Second wave | 144 (36.1) | 53 (32.7) | 48 (37.8) | 43 (39.4) | 0.474 |
| Third wave | 187 (47.0) | 82 (50.6) | 56 (44.0) | 49 (45.0) | 0.480 |
| Time from hospital to ICU admission, days, median (IQR) | 3 (0–3) | 3.2 (0–4) | 1.7 (0–2) | 2.9 (0–3) | 0.68 |
| IMV, n (%) | 76 (19.1) | 29 (17.9) | 27 (21.3) | 20 (18.3) | 0.93 |
| ECMO, n (%) | 78 (19.6) | 22 (13.6) | 18 (14.2) | 38 (34.9) | <0.001 |
| Antiviral agent a, n (%) | 134 (33.7) | 43 (26.5) | 46 (36.2) | 45 (41.3) | 0.032 |
| Antibiotic treatment, n (%) | 264 (66.3) | 114 (70.4) | 80 (63) | 70 (64.2) | 0.21 |
| Documented co-infection, n (%) | 44 (11.1) | 12 (7.4) | 13 (10.2) | 19 (17.4) | 0.035 |
| TOTAL (n = 398) | 0 VAP (n = 162) | 1 VAP (n = 127) | >1 VAP (n = 109) | |
|---|---|---|---|---|
| IS therapy, n (%) | 338 (84.9) | 129 (79.6) a | 108 (85) | 101 (92.7) b |
| Dexamethasone, n (%) | 324 (81.4) | 129 (79.6) | 103 (81.1) | 92 (84.4) |
| Methylprednisolone, n (%) | 104 (26.1) | 25 (15.4) a | 26 (20.5) a | 53 (48.6) b,c |
| IL-1 receptor antagonist, n (%) | 15 (3.8) | 4 (2.5) | 4 (3.1) | 7 (6.4) |
| JAK receptor antagonist, n (%) | 19 (4.8) | 4 (2.5) | 8 (6.3) | 7 (6.4) |
| IL-6 receptor antagonist, n (%)c | 75 (18.8) | 12 (7.4) a,c | 31 (24.4) b | 32 (29.4) b |
| Combination of 2 IS, n (%)d | 193 (48.5) | 51 (31.5)a,c | 63 (49.6) a,b | 79 (72.5) b,c |
| 1st VAP (n = 338) | 2nd VAP (n = 165) | 3rd VAP (n = 69) | |
|---|---|---|---|
| Gram-negative bacilli, n (%) | 189 (55.9) | 101 (61.2) | 48 (69.6) |
| Enterobacteriaceae | 139 | 60 | 22 |
| Non-fermenting GNB | 50 | 41 | 26 |
| Gram-positive cocci, n (%) | 87 (25.7) | 31 (18.8) | 8 (11.6) |
| MSSA | 50 | 21 | 5 |
| MRSA | 6 | 3 | 1 |
| Enterococcus spp. | 14 | 6 | 2 |
| Streptococcus spp. | 17 | 1 | 0 |
| Polymicrobial, n (%) | 62 (18.3) | 33 (20.0) | 13 (18.9) |
| Antibiotic-multiresistant bacteria, n | 11 (3.2) | 14 (8.5) | 14 (20.0) |
| ESBLE-producing Enterobacteriaceae | 8 | 11 | 10 |
| Carbapenem-resistant enterobacteriaceae | 1 | 2 | 1 |
| Multi-drug resistant Pseudomonas | 2 | 1 | 3 |
| TOTAL (n = 398) | 0 VAP (n = 162) | 1 VAP (n = 127) | >1 VAP (n = 109) | p | |
|---|---|---|---|---|---|
| OUTCOMES, days, median (IQR) | |||||
| Duration of mechanical ventilation | 17 (8–36) | 10 (5–18) | 16 (8–30) | 41 (25–56) | <0.001 |
| VFD at D28 | 9 (0–19) | 17 (8–22) | 11 (0–19) | 0 (0–1) | <0.001 |
| VFD at D60 | 41 (21–51) | 48 (40–54) | 42.5 (28–51) | 17 (0–33) | <0.001 |
| ICU length of stay | 23 (12–42) | 14 (9–25) | 22 (12–36) | 46 (29–66) | <0.001 |
| Hospital length of stay | 29 (18–49) | 22 (14–36) | 29 (17–44) | 53 (32–75) | <0.001 |
| MORTALITY OUTCOMES, n (%) | |||||
| ICU mortality | 111 (27.9) | 32 (19.8) | 43 (33.9) | 36 (33.0) | 0.011 |
| D28 mortality | 69 (17.3) | 30 (18.5) | 30 (23.6) | 9 (8.3) | 0.006 |
| D90 mortality | 114 (28.6) | 34 (21.0) | 46 (36.2) | 34 (31.2) | 0.021 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| 1 VAP (n = 127) | Recurrence (n = 109) | p | Odds Ratio | 95% CI | p | |
| Variable | ||||||
| Age, y | 63 ± 12 | 64 ± 10 | 0.54 | |||
| SAPS 2 | 40 (33–48) | 42 (34–51) | 0.13 | 1 | 0.98–1.03 | 0.62 |
| SOFA a | 5 (3–8) | 6 (4–8) | 0.20 | |||
| Obesity, n (%) | 43 (34) | 46 (42) | 0.22 | |||
| IS treatment (at least one), n (%) | 106 (83) | 101 (93) | 0.06 | 0.5 | 0.18–1.39 | 0.19 |
| Steroidal IS, n (%) | 42 (33) | 74 (68) | <0.001 | 0.75 | 0.37–1.52 | 0.43 |
| Non steroidal IS, n (%) | 39 (31) | 39 (36) | 0.46 | |||
| Association of 2 IS, n (%) | 62 (49) | 79 (73) | <0.001 | 0.66 | 0.34–1.27 | 0.21 |
| Bacterial co-infection at ICU admission, n (%) | 12 (9) | 19 (17) | 0.08 | 0.64 | 0.26–1.56 | 0.32 |
| Antibiotic target attainment b | 20 (69) | 30 (75) | 0.07 | 0.94 | 0.43–2.09 | 0.89 |
| Duration of MV | 16 (8–30) | 41 (25–56) | <0.001 | 1.06 | 1.04–1.08 | <0.001 |
| First VAP documentation | ||||||
| Gram positive Cocci | 51 | 42 | 0.72 | |||
| Enterobacteriaceae | 60 | 61 | 0.22 | |||
| Non-fermenting negative Gram Bacilli | 24 | 23 | 0.72 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gragueb-Chatti, I.; Hyvernat, H.; Leone, M.; Agard, G.; Peres, N.; Guervilly, C.; Boucekine, M.; Hamidi, D.; Papazian, L.; Dellamonica, J.; et al. Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study. J. Clin. Med. 2022, 11, 7097. https://doi.org/10.3390/jcm11237097
Gragueb-Chatti I, Hyvernat H, Leone M, Agard G, Peres N, Guervilly C, Boucekine M, Hamidi D, Papazian L, Dellamonica J, et al. Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study. Journal of Clinical Medicine. 2022; 11(23):7097. https://doi.org/10.3390/jcm11237097
Chicago/Turabian StyleGragueb-Chatti, Ines, Hervé Hyvernat, Marc Leone, Geoffray Agard, Noémie Peres, Christophe Guervilly, Mohamed Boucekine, Dany Hamidi, Laurent Papazian, Jean Dellamonica, and et al. 2022. "Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study" Journal of Clinical Medicine 11, no. 23: 7097. https://doi.org/10.3390/jcm11237097
APA StyleGragueb-Chatti, I., Hyvernat, H., Leone, M., Agard, G., Peres, N., Guervilly, C., Boucekine, M., Hamidi, D., Papazian, L., Dellamonica, J., Lopez, A., & Hraiech, S. (2022). Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study. Journal of Clinical Medicine, 11(23), 7097. https://doi.org/10.3390/jcm11237097








