Clinical Usability of Embryo Development Using a Combined Qualitative and Quantitative Approach in a Single Vitrified-Warmed Blastocyst Transfer: Assessment of Pre-Vitrified Blastocyst Diameter and Post-Warmed Blastocyst Re-Expansion Speed
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Endometrial Preparation
2.3. Vitrified-Warmed Blastocyst Preparation
2.4. Recording and Annotation of Blastocyst Culture in the TLS
2.5. Measurements of Blastocyst Diameter and Re-Expansion Speed
2.6. Blastocyst Survival, Re-Expansion, and Grading Assessment
2.7. Embryo Transfer and Identification of Outcome Parameters
2.8. Statistical Analysis
3. Results
3.1. General Characteristics of the Patients
3.2. Laboratory and Clinical Outcomes
3.2.1. Phase 1: Diameter
3.2.2. Phase 2: Re-Expansion Speed
3.3. Multivariate Logistic Regression Analysis of Blastocyst Quality
3.4. Multivariate Logistic Regression Analysis of Ongoing Pregnancy
3.5. Clinical and Ongoing Pregnancy Based on Quantitative (Speed) and Qualitative (BQS) Selection Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boucret, L.; Tramon, L.; Saulnier, P.; Ferré-L’Hôtellier, V.; Bouet, P.E.; May-Panloup, P. Change in the Strategy of Embryo Selection with Time-Lapse System Implementation-Impact on Clinical Pregnancy Rates. J. Clin. Med. 2021, 10, 4111. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.; Park, H. Time-lapse technology for embryo culture and selection. Upsala J. Med. Sci. 2020, 125, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Kim, M.K.; Kim, T.H.; Kim, J.W.; Chang, E.M.; Lyu, S.W.; Kim, J.Y.; Lee, W.S. Association between different dual trigger dosages and in vitro fertilization results in patients with patient-oriented strategies encompassing individualized oocyte number group IV. Obstet. Gynecol. Sci. 2022, 65, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Meseguer, M.; Rubio, C.; Treff, N.R. Diagnosis of human preimplantation embryo viability. Hum. Reprod. Update 2015, 21, 727–747. [Google Scholar] [CrossRef]
- Kirkegaard, K.; Ahlström, A.; Ingerslev, H.J.; Hardarson, T. Choosing the best embryo by time-lapse versus standard morphology. Fertil. Steril. 2015, 103, 323–332. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishikawa, H.; Ishii, K.; Sato, A.; Nakamura, N.; Saito, Y.; Hasegawa, H.; Fujita, M.; Mitsuhashi, A.; Shozu, M. Time-lapse monitoring of fertilized human oocytes focused on the incidence of 0PN embryos in conventional in vitro fertilization cycles. Sci. Rep. 2021, 11, 18862. [Google Scholar] [CrossRef]
- Lee, A.M.; Connell, M.T.; Csokmay, J.M.; Styer, A.K. Elective single embryo transfer-the power of one. Contracept. Reprod. Med. 2016, 1, 11. [Google Scholar] [CrossRef]
- Sullivan, E.A.; Wang, Y.A.; Hayward, I.; Chambers, G.M.; Illingworth, P.; McBain, J.; Norman, R.J. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum. Reprod. 2012, 27, 3609–3615. [Google Scholar] [CrossRef]
- Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine. Elective single-embryo transfer. Fertil. Steril. 2012, 97, 835–842. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.K.; Eum, J.H.; Song, H.; Lee, W.S.; Lyu, S.W. Embryo Selection Based on Morphological Parameters in a Single Vitrified-Warmed Blastocyst Transfer Cycle. Reprod. Sci. 2021, 28, 1060–1068. [Google Scholar] [CrossRef]
- Du, Q.Y.; Wang, E.Y.; Huang, Y.; Guo, X.Y.; Xiong, Y.J.; Yu, Y.P.; Yao, G.D.; Shi, S.L.; Sun, Y.P. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil. Steril. 2016, 105, 910–919.e1. [Google Scholar] [CrossRef] [PubMed]
- Nazem, T.G.; Sekhon, L.; Lee, J.A.; Overbey, J.; Pan, S.; Duke, M.; Briton-Jones, C.; Whitehouse, M.; Copperman, A.B.; Stein, D.E. The correlation between morphology and implantation of euploid human blastocysts. Reprod. Biomed. Online 2019, 38, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Y.; Huang, X.; Sun, L.; Li, Y. Blastocoele expansion: An important parameter for predicting clinical success pregnancy after frozen-warmed blastocysts transfer. Reprod. Biol. Endocrinol. 2019, 17, 15. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Capalbo, A.; Levi-Setti, P.E.; Soscia, D.; Orlando, G.; Albani, E.; Parini, V.; Stoppa, M.; Dovere, L.; Tacconi, L.; et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum. Reprod. 2018, 33, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Warshaviak, M.; Kalma, Y.; Carmon, A.; Samara, N.; Dviri, M.; Azem, F.; Ben-Yosef, D. The Effect of Advanced Maternal Age on Embryo Morphokinetics. Front. Endocrinol. 2019, 10, 686. [Google Scholar] [CrossRef]
- Minasi, M.G.; Colasante, A.; Riccio, T.; Ruberti, A.; Casciani, V.; Scarselli, F.; Spinella, F.; Fiorentino, F.; Varricchio, M.T.; Greco, E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: A consecutive case series study. Hum. Reprod. 2016, 31, 2245–2254. [Google Scholar] [CrossRef]
- Armstrong, S.; Bhide, P.; Jordan, V.; Pacey, A.; Marjoribanks, J.; Farquhar, C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst. Rev. 2019, 5, CD011320. [Google Scholar] [CrossRef]
- Ueno, S.; Uchiyama, K.; Kuroda, T.; Okimura, T.; Yabuuchi, A.; Kobayashi, T.; Kato, K. Establishment of day 7 blastocyst freezing criteria using blastocyst diameter for single vitrified-warmed blastocyst transfer from live birth outcomes: A single-center, large cohort, retrospectively matched study. J. Assist. Reprod. Genet. 2020, 37, 2327–2335. [Google Scholar] [CrossRef]
- Huang, T.T.; Huang, D.H.; Ahn, H.J.; Arnett, C.; Huang, C.T. Early blastocyst expansion in euploid and aneuploid human embryos: Evidence for a non-invasive and quantitative marker for embryo selection. Reprod. Biomed. Online 2019, 39, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Meseguer, M. Focus on time-lapse analysis: Blastocyst collapse and morphometric assessment as new features of embryo viability. Reprod. Biomed. Online 2021, 43, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Lagalla, C.; Barberi, M.; Orlando, G.; Sciajno, R.; Bonu, M.A.; Borini, A. A quantitative approach to blastocyst quality evaluation: Morphometric analysis and related IVF outcomes. J. Assist. Reprod. Genet. 2015, 32, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Almagor, M.; Harir, Y.; Fieldust, S.; Or, Y.; Shoham, Z. Ratio between inner cell mass diameter and blastocyst diameter is correlated with successful pregnancy outcomes of single blastocyst transfers. Fertil. Steril. 2016, 106, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Cevik, M. Correlation between the cryosurvival, cell number and diameter in bovine in vitro produced embryos. Cryobiology 2016, 73, 203–208. [Google Scholar] [CrossRef]
- Van Marion, E.S.; Chavli, E.A.; Laven, J.S.E.; Steegers-Theunissen, R.P.M.; Koster, M.P.H.; Baart, E.B. Longitudinal surface measurements of human blastocysts show that the dynamics of blastocoel expansion are associated with fertilization method and ongoing pregnancy. Reprod. Biol. Endocrinol. 2022, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Park, J.K.; Kim, T.H.; Eum, J.H.; Song, H.; Kim, J.Y.; Park, H.M.; Park, C.W.; Lee, W.S.; Lyu, S.W. The impact of post-warming culture duration on clinical outcomes of vitrified-warmed single blastocyst transfer cycles. Clin. Exp. Reprod. Med. 2020, 47, 312–318. [Google Scholar] [CrossRef]
- Lee, H.N.; Park, J.K.; Paek, S.K.; Byun, J.H.; Song, H.; Lee, H.J.; Chang, E.M.; Kim, J.W.; Lee, W.S.; Lyu, S.W. Does duration of cryostorage affect survival rate, pregnancy, and neonatal outcomes? Large-scale single-center study of slush nitrogen (SN2) vitrified-warmed blastocysts. Int. J. Gynaecol. Obstet. 2021, 152, 351–357. [Google Scholar] [CrossRef]
- Ebner, T.; Oppelt, P.; Radler, E.; Allerstorfer, C.; Habelsberger, A.; Mayer, R.B.; Shebl, O. Morphokinetics of vitrified and warmed blastocysts predicts implantation potential. J. Assist. Reprod. Genet. 2017, 34, 239–244. [Google Scholar] [CrossRef]
- Inoue, T.; Taguchi, S.; Uemura, M. Migration speed of nucleolus precursor bodies in human male pronuclei: A novel parameter for predicting live birth. J. Assist. Reprod. Genet. 2021, 38, 1725–1736. [Google Scholar] [CrossRef]
- Park, J.K.; Ahn, S.Y. Does Post-Warming Extended Culture Duration Affect the Clinical and Obstetric Outcomes of Patients of Advanced Maternal Age? A Single-Center Study. J. Korean Med. Sci. 2022, 37, e96. [Google Scholar] [CrossRef]
- Sciorio, R.; Thong, D.; Thong, K.J.; Pickering, S.J. Clinical pregnancy is significantly associated with the blastocyst width and area: A time-lapse study. J. Assist. Reprod. Genet. 2021, 38, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, O. Abstracts of the 34th Annual Meeting of the European Society of Human Reproduction and Embryology. Hum. Reprod. 2018, 33, i1–i541. [Google Scholar]
- Coello, A.; Meseguer, M.; Galán, A.; Alegre, L.; Remohí, J.; Cobo, A. Analysis of the morphological dynamics of blastocysts after vitrification/warming: Defining new predictive variables of implantation. Fertil. Steril. 2017, 108, 659–666.e654. [Google Scholar] [CrossRef]
- Ahlström, A.; Westin, C.; Wikland, M.; Hardarson, T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum. Reprod. 2013, 28, 1199–1209. [Google Scholar] [CrossRef]
- Shu, Y.; Watt, J.; Gebhardt, J.; Dasig, J.; Appling, J.; Behr, B. The value of fast blastocoele re-expansion in the selection of a viable thawed blastocyst for transfer. Fertil. Steril. 2009, 91, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Giunco, H.; Connerney, M.; Boylan, C.; Koelper, N.; Mersereau, J.; Berger, D.S. Embryo re-expansion does not affect clinical pregnancy rates in frozen embryo transfer cycles: A retrospective study. J. Assist. Reprod. Genet. 2021, 38, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Kovačič, B.; Taborin, M.; Vlaisavljević, V. Artificial blastocoel collapse of human blastocysts before vitrification and its effect on re-expansion after warming—A prospective observational study using time-lapse microscopy. Reprod. Biomed. Online 2018, 36, 121–129. [Google Scholar] [CrossRef]
- Esfandiari, N.; Dela Cruz, D.B.; Rao, K.; Casper, R. Re-expansion of warmed vitrified blastocysts before embryo transfer; are we too concerned? Fertil. Steril. 2016, 106, e137. [Google Scholar] [CrossRef]
- Lin, R.; Feng, G.; Shu, J.; Zhang, B.; Zhou, H.; Gan, X.; Wang, C.; Chen, H. Blastocoele re-expansion time in vitrified-warmed cycles is a strong predictor of clinical pregnancy outcome. J. Obstet. Gynaecol. 2017, 43, 689–695. [Google Scholar] [CrossRef]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef]
- Rehman, K.S.; Bukulmez, O.; Langley, M.; Carr, B.R.; Nackley, A.C.; Doody, K.M.; Doody, K.J. Late stages of embryo progression are a much better predictor of clinical pregnancy than early cleavage in intracytoplasmic sperm injection and in vitro fertilization cycles with blastocyst-stage transfer. Fertil. Steril. 2007, 87, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Long, H.; Gao, H.; Guo, W.; Xie, Y.; Chen, D.; Cong, Y.; Wang, Y.; Li, D.; Si, J.; et al. The Valuable Reference of Live Birth Rate in the Single Vitrified-Warmed BB/BC/CB Blastocyst Transfer: The Cleavage-Stage Embryo Quality and Embryo Development Speed. Front. Physiol. 2020, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, S.; Liu, J.; Kang, X.; Liu, H. Effect of blastocyst morphology and developmental speed on transfer strategy for grade “C” blastocyst in vitrified-warmed cycles. J. Ovarian Res. 2021, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, J.K.; Jeon, Y.; Choe, S.A.; Lee, H.J.; Kim, J.; Chang, E.M.; Kim, J.W.; Lyu, S.W.; Kim, J.Y.; et al. Correlation between Morphologic Grading and Euploidy Rates of Blastocysts, and Clinical Outcomes in In Vitro Fertilization Preimplantation Genetic Screening. J. Korean Med. Sci. 2019, 34, e27. [Google Scholar] [CrossRef] [PubMed]
- Hershko-Klement, A.; Raviv, S.; Nemerovsky, L.; Rom, T.; Itskovich, A.; Bakhshi, D.; Shulman, A.; Ghetler, Y. Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction. J. Clin. Med. 2022, 11, 2673. [Google Scholar] [CrossRef]
- Kato, K.; Ueno, S.; Yabuuchi, A.; Uchiyama, K.; Okuno, T.; Kobayashi, T.; Segawa, T.; Teramoto, S. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod. Biomed. Online 2014, 29, 411–416. [Google Scholar] [CrossRef]
- Aghajanova, L.; Hamilton, A.E.; Giudice, L.C. Uterine receptivity to human embryonic implantation: Histology, biomarkers, and transcriptomics. Semin. Cell Dev. Biol. 2008, 19, 204–211. [Google Scholar] [CrossRef]
- Ruiz-Alonso, M.; Galindo, N.; Pellicer, A.; Simón, C. What a difference two days make: “personalized” embryo transfer (pET) paradigm: A case report and pilot study. Hum. Reprod. 2014, 29, 1244–1247. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Richter, K.S.; Harris, D.C.; Daneshmand, S.T. Influence of patient age on the growth and transfer of blastocyst-stage embryos. Fertil. Steril. 2002, 77, 700–705. [Google Scholar] [CrossRef]
- Desai, N.; Ploskonka, S.; Goodman, L.; Attaran, M.; Goldberg, J.M.; Austin, C.; Falcone, T. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil. Steril. 2016, 106, 1370–1378. [Google Scholar] [CrossRef]

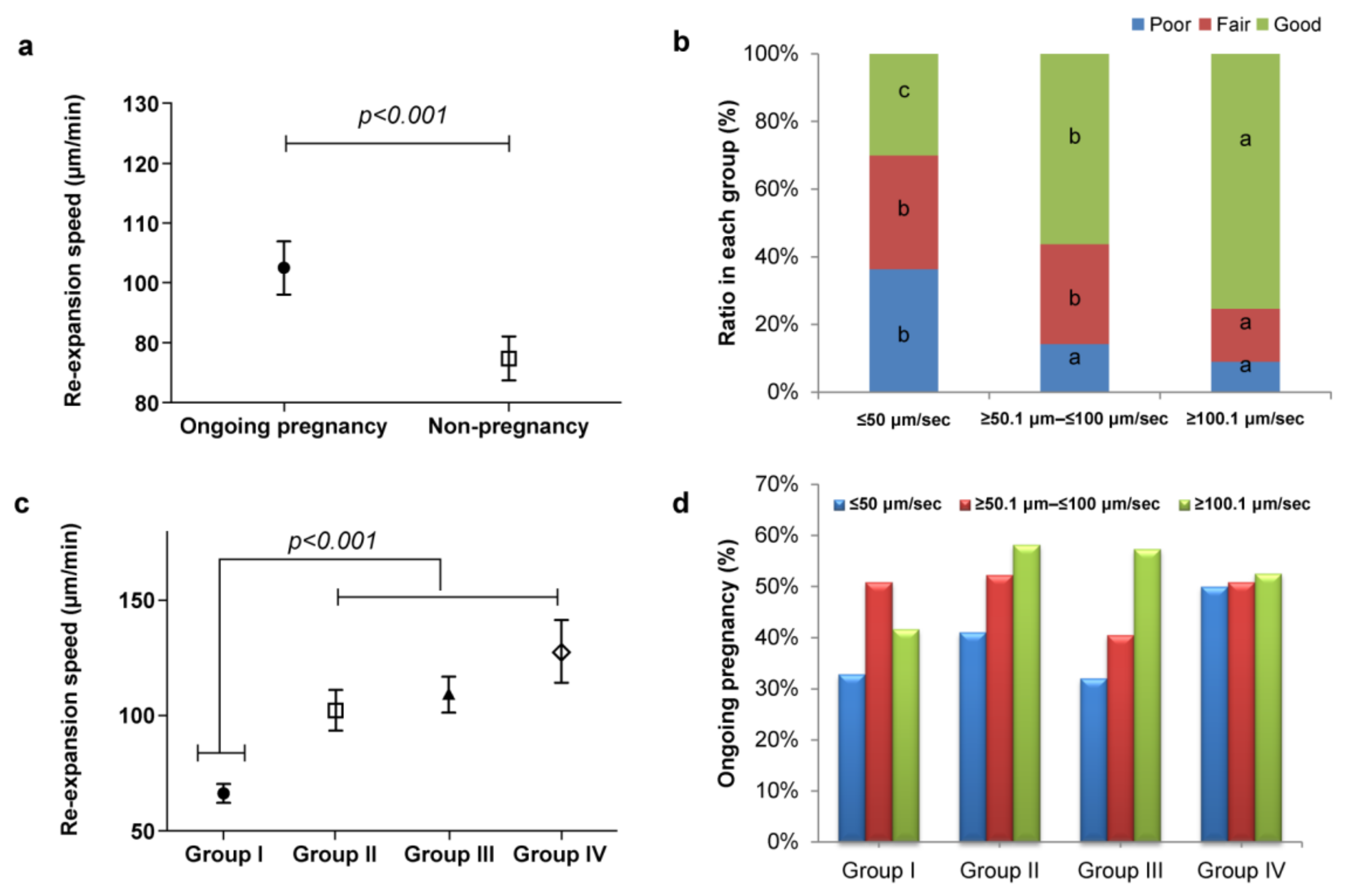

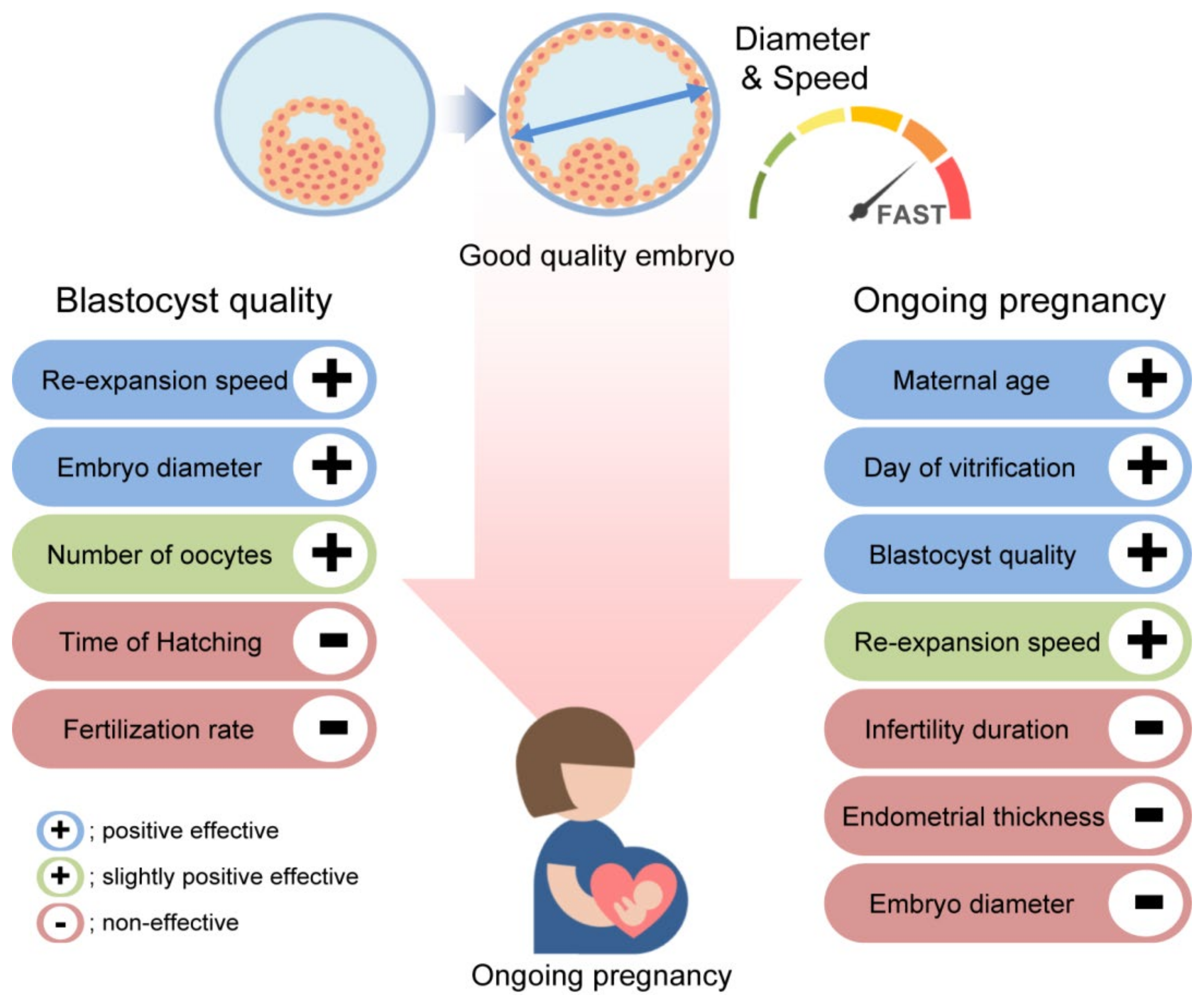
| Characteristics | Variables | All Transfer | Group I | Group II | Group III | Group IV | p-Value |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | Number of FET cycles | 658 | 158 | 197 | 171 | 132 | |
| Cryo survival rate | 98.35 ± 0.09 | 96.60 ± 0.12 | 98.89 ± 0.08 | 98.93 ± 0.07 | 98.86 ± 0.07 | 0.24 | |
| Mean maternal age (years, ±SD) | 33.93 ± 3.59 | 33.97 ± 4.13 | 33.67 ± 3.38 | 34.14 ± 3.63 | 33.97 ± 3.16 | 0.65 | |
| Mean paternal age (years, ±SD) | 36.98 ± 4.54 | 37.53 ± 5.69 | 36.90 ± 4.28 | 37.01 ± 4.06 | 36.44 ± 3.93 | 0.25 | |
| Etiology of infertility [(%) n] | |||||||
| Female | 40.9% (269) | 41.1% (65) a | 32.5% (64) a,b | 42.7% (73) a | 50.8% (67) a,c | 0.01 | |
| Male | 19.0% (125) | 13.9% (22) | 24.4% (48) | 21.1% (36) | 14.4% (19) | 0.08 | |
| Combine (male + female) | 25.5% (168) | 30.4% (48) | 29.4% (58) | 21.1% (36) | 19.7% (26) | 0.06 | |
| Unexplained | 12.2% (80) | 10.1% (16) | 11.2% (22) | 13.5% (23) | 14.4% (19) | 0.64 | |
| Other | 2.4% (16) | 4.4% (7) | 2.5% (5) | 1.8% (3) | 0.8% (1) | 0.21 | |
| EM thickness at hCG (mm) | 9.59 ± 1.70 | 9.61 ± 1.82 | 9.71 ± 1.76 | 9.51 ± 1.56 | 9.48 ± 1.66 | 0.58 | |
| FET cycle protocol | |||||||
| Natural [(%) n] | 81.6% (537) | 79.7% (126) | 85.3% (168) | 80.1% (1370 | 80.3% (106) | 0.47 | |
| HRT [(%) n] | 18.4% (121) | 20.3% (32) | 14.7% (29) | 19.9% (34) | 19.7% (26) | 0.35 | |
| Characteristics of previous IVF cycle | Maternal age at oocyte retrieval (years, ±SD) | 33.52 ± 3.61 | 33.97 ± 4.13 | 33.13 ± 3.37 | 33.62 ± 3.66 | 33.48 ± 3.16 | 0.18 |
| Paternal age at oocyte retrieval (years, ±SD) | 36.49 ± 4.42 | 37.24 ± 5.36 | 36.32 ± 4.21 | 36.41 ± 4.03 | 36.00 ± 3.92 | 0.10 | |
| Infertility duration (y) | 3.19 ± 2.2 | 3.14 ± 2.27 | 3.07 ± 2.11 | 3.4 ± 2.34 | 3.15 ± 2.07 | 0.62 | |
| Number of previous IVF attempts | 1.54 ± 1.02 | 1.63 ± 1.30 | 1.52 ± 0.94 | 1.62 ± 1.01 | 1.35 ± 0.74 | 0.09 | |
| BMI (kg/m2) | 21.13 ± 2.65 | 21.35 ± 2.77 | 20.98 ± 2.51 | 21.23 ± 2.80 | 20.97 ± 2.53 | 0.52 | |
| Parity | 0.10 ± 0.33 | 0.09 ± 0.29 | 0.09 ± 0.36 | 0.12 ± 0.35 | 0.08 ± 0.30 | 0.70 | |
| AMH (ng/mL) | 4.26 ± 3.47 | 4.26 ± 3.77 | 4.51 ± 3.53 | 4.33 ± 3.70 | 3.81 ± 2.62 | 0.37 | |
| Basal FSH (IU/L) | 7.19 ± 2.97 | 7.54 ± 3.25 | 7.16 ± 3.35 | 6.85 ± 2.54 | 7.26 ± 2.48 | 0.23 | |
| Basal LH (IU/L) | 5.97 ± 3.60 | 5.81 ± 3.16 | 5.97 ± 3.62 | 5.88 ± 4.07 | 6.26 ± 3.46 | 0.76 | |
| Basal E2 (IU/L) | 44.32 ± 22.19 | 44.49 ± 18.51 | 43.88 ± 17.61 | 45.93 ± 32.24 | 42.72 ± 15.98 | 0.68 | |
| Basal TSH (mIU/mL) | 1.56 ± 1.24 | 1.42 ± 0.81 | 1.62 ± 1.32 | 1.60 ± 1.39 | 1.58 ± 1.33 | 0.47 | |
| Basal Prolactin (ng/mL) | 15.51 ±10.04 10.0401 | 15.69 ± 11.826 | 15.01 ± 9.40 | 15.965 ± 10.133 | 15.48 ± 8.51 | 0.84 | |
| MCD-AFC | 16.66 ± 9.66 | 15.33 ± 10.63 | 17.81 ± 8.52 | 17.20 ± 10.9 | 15.84 ± 8.21 | 0.09 | |
| Ovarian stimulation protocol | |||||||
| Antagonist [(%) n] | 93.0% (612) | 93.7% (148) | 93.4% (184) | 90.6% (155) | 94.7% (125) | 0.53 | |
| Agonist [(%) n] | 3.2% (21) | 3.2% (5) | 3.6% (7) | 4.1% (7) | 1.5% (2) | 0.63 | |
| Natural [(%) n] | 3.8% (25) | 3.2% (5) | 3.0% (6) | 5.3% (9) | 3.8% (5) | 0.69 | |
| FSH total dose | 1503.9 ± 469.94 | 1521.1 ± 475.66 | 1458.1 ± 439.13 | 1518.6 ± 484.49 | 1533.8 ± 489.93 | 0.48 | |
| Fertilization method | |||||||
| Standard IVF [(%) n] | 31.3% (206) | 26.6% (42) a | 22.8% (45) a | 31.0% (53) a | 50.0% (66) b | 0.01 | |
| ICSI [(%) n] | 60.2% (396) | 63.9% (101) a | 71.1% (140) a | 60.8% (104) a | 38.6% (51) b | 0.01 | |
| Half ICSI [(%) n] | 8.5% (56) | 9.5% (15) | 6.1% (12) | 8.2% (14) | 11.4% (15) | 0.38 | |
| Vitrification total | |||||||
| Surplus [(%) n] | 25.8% (170) | 17.1% (27) a | 26.4% (52) a | 25.1% (43) a | 36.4% (48) a,b | 0.01 | |
| All freezing [(%) n] | 74.2% (488) | 82.9% (131) a | 73.6% (145) a | 74.9% (128) a | 63.6% (84) a,b | 0.01 | |
| Mean number of retrieved oocytes (n) | 16.64 ± 9.04 | 16.44 ± 9.61 | 16.95 ± 9.12 | 16.82 ± 9.15 | 16.19 ± 8.12 | 0.88 | |
| Fertilization embryos (2 pronuclei) | 11.05 ± 6.1 | 10.71 ± 6.59 | 11.03 ± 5.72 | 11.2 ± 6.19 | 11.3 ± 5.98 | 0.85 | |
| Mean number of frozen blastocysts (n) | 3.32 ± 2.4 | 3.23 ± 2.32 | 3.26 ± 2.31 | 3.35 ± 2.44 | 3.44 ± 2.55 | 0.89 | |
| Day of vitrification [(%) n] | |||||||
| D5 | 86.9% (572) | 92.4% (146) a | 92.4% (182) a | 88.9% (152) a | 69.7% (92) b | 0.01 | |
| D6 | 13.1% (86) | 7.6% (12) a | 7.6% (15) a | 11.1% (19) a | 30.3% (40) b | 0.01 | |
| Variables | OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Maternal age at oocyte retrieval (years) | ||||
| ≤30 | 1.070 (0.609–1.879) | 0.814 | n/a | |
| 31–34 | 1.252 (0.802–1.955) | 0.322 | n/a | |
| ≥35 | Reference | |||
| Embryo diameter (μm) | ||||
| <135 | Reference | Reference | ||
| ≥135–≤145 | 4.801 (2.888–7.982) | 0.000 | 2.815 (1.567–5.056) * | 0.001 |
| ≥146–≤155 | 9.668 (5.063–18.460) | 0.000 | 5.315 (2.584–10.934) * | 0.000 |
| ≥156 | 12.330 (5.647–26.919) | 0.000 | 7.917 (3.113–20.135) * | 0.000 |
| Embryo re-expansion speed (μm/min) | ||||
| ≤50 | Reference | Reference | ||
| ≥50.1–≤100 | 3.443 (2.141–5.534) | 0.000 | 1.774 (0.994–3.167) * | 0.053 |
| ≥100.1–≤150 | 5.774 (3.228–10.330) | 0.000 | 2.484 (1.232–5.008) * | 0.011 |
| tHSP (hatching start point) (h) | ||||
| ≤4 | 2.959 (1.910–4.586) | 0.000 | n/a | |
| ≥4.1 | Reference | |||
| Number of retrieved oocytes | ||||
| ≤10 | Reference | Reference | ||
| ≥11–≤20 | 1.823 (1.108–2.999) | 0.018 | 2.019 (1.122–3.632) * | 0.019 |
| ≥21 | 1.311 (0.789–2.179) | 0.295 | n/a | |
| Number of fertilization embryos | ||||
| ≤7 | Reference | |||
| ≥ 8–≤16 | 1.465 (0.936–2.294) | 0.950 | n/a | |
| ≥17 | 1.810 (0.951–3.444) | 0.071 | n/a | |
| Variables | OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Maternal age at ET (years) | ||||
| <38 | 2.170 (1.414–3.331) | 0.001 | 1.939 (1.245–3.014) * | 0.003 |
| ≥38 | Reference | Reference | ||
| Infertility duration (years) | ||||
| ≤2 | 1.152 (0.077–1.722) | 0.490 | n/a | |
| ≥3–≤4 | 1.013 (0.656–1.565) | 0.953 | n/a | |
| ≥5 | Reference | n/a | ||
| Endometrial thickness in VBT (mm) | ||||
| <10 | 0.766 (0.563–1.043) | 0.091 | n/a | |
| ≥10 | Reference | n/a | ||
| Day of vitrification | ||||
| Day 5 | 1.846 (1.146–2.971) | 0.012 | 2.027 (1.237–3.311) * | 0.005 |
| Day 6 | Reference | Reference | ||
| Blastocyst quality score | ||||
| good | 0.543 (0.359–0.822) | 0.004 | 0.641 (0.411–0.999) * | 0.050 |
| poor | Reference | Reference | ||
| Embryo diameter (μm) | ||||
| <135 | Reference | n/a | ||
| ≥135–≤145 | 1.629 (1.066–2.490) | 0.024 | n/a | |
| ≥146–≤155 | 1.268 (0.818–1.967) | 0.289 | n/a | |
| ≥156 | 1.548 (0.970–2.471) | 0.067 | n/a | |
| Embryo re-expansion speed (μm/min) | ||||
| ≤50 | Reference | Reference | ||
| ≥50.1–≤100 | 1.633 (1.082-2.464) | 0.020 | n/a | |
| ≥100.1 | 2.096 (1.356-3.240) | 0.001 | 1.931 (1.159–2.994) * | 0.010 |
| AUROC | 95% CI | Optimal Cutoff Value | Sensitivity | Specificity | PPV | NPV | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Speed | 0.599 | 0.556–0.641 | 59.8 | 0.75 | 0.38 | 0.53 | 0.37 | <0.0001 |
| BQS | 0.592 | 0.549–0.635 | 7 | 0.78 | 0.39 | 0.53 | 0.33 | <0.0001 |
| Time-Lapse Selection Criteria | Applicable | Not Applicable | p-Value |
|---|---|---|---|
| No. of single blastocysts transferred | 340 | 95 | |
| No. of clinical pregnancy [n (%)] | 212 (62.40%) | 31 (32.60%) | <0.0001 |
| No. of abortions [n (%)] | 22 (6.50%) | 5 (5.30%) | NS |
| No. of ongoing pregnancy [n (%)] | 190 (55.90%) | 26 (27.40%) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.K.; Ahn, S.-Y.; Seok, S.H.; Park, S.Y.; Bang, S.; Eum, J.H.; Kwak, I.P.; Kim, J.W.; Lee, W.S. Clinical Usability of Embryo Development Using a Combined Qualitative and Quantitative Approach in a Single Vitrified-Warmed Blastocyst Transfer: Assessment of Pre-Vitrified Blastocyst Diameter and Post-Warmed Blastocyst Re-Expansion Speed. J. Clin. Med. 2022, 11, 7085. https://doi.org/10.3390/jcm11237085
Park JK, Ahn S-Y, Seok SH, Park SY, Bang S, Eum JH, Kwak IP, Kim JW, Lee WS. Clinical Usability of Embryo Development Using a Combined Qualitative and Quantitative Approach in a Single Vitrified-Warmed Blastocyst Transfer: Assessment of Pre-Vitrified Blastocyst Diameter and Post-Warmed Blastocyst Re-Expansion Speed. Journal of Clinical Medicine. 2022; 11(23):7085. https://doi.org/10.3390/jcm11237085
Chicago/Turabian StylePark, Jae Kyun, So-Yeon Ahn, Su Hee Seok, Sol Yi Park, Soyoung Bang, Jin Hee Eum, In Pyung Kwak, Ji Won Kim, and Woo Sik Lee. 2022. "Clinical Usability of Embryo Development Using a Combined Qualitative and Quantitative Approach in a Single Vitrified-Warmed Blastocyst Transfer: Assessment of Pre-Vitrified Blastocyst Diameter and Post-Warmed Blastocyst Re-Expansion Speed" Journal of Clinical Medicine 11, no. 23: 7085. https://doi.org/10.3390/jcm11237085
APA StylePark, J. K., Ahn, S.-Y., Seok, S. H., Park, S. Y., Bang, S., Eum, J. H., Kwak, I. P., Kim, J. W., & Lee, W. S. (2022). Clinical Usability of Embryo Development Using a Combined Qualitative and Quantitative Approach in a Single Vitrified-Warmed Blastocyst Transfer: Assessment of Pre-Vitrified Blastocyst Diameter and Post-Warmed Blastocyst Re-Expansion Speed. Journal of Clinical Medicine, 11(23), 7085. https://doi.org/10.3390/jcm11237085






