The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Pacing Procedure
2.4. Echocardiographic Parameters
2.5. Follow-Up
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Procedural Characteristics
3.2. Echocardiographic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dandamudi, G.; Vijayaraman, P. How to perform permanent His bundle pacing in routine clinical practice. Heart Rhythm. 2016, 13, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Ellenbogen, K.A.; Dandamudi, G.; Sharma, P.S.; Huang, W.; Lustgarten, D.L.; Tung, R.; Tada, H.; Koneru, J.N.; Bergemann, T.; et al. Permanent His-Bundle pacing: A systematic literature review and meta-analysis. Europace 2018, 20, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.G.; Musat, D.L.; Milstein, N.; Pimienta, J.; Flynn, L.; Sichrovsky, T.; Preminger, M.W.; Mittal, S. The Efficacy of His Bundle Pacing: Lessons Learned from Implementation for the First Time at an Experienced Electrophysiology Center. JACC Clin. Electrophysiol. 2018, 4, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, C.; Auquier, N.; Milhem, A.; Mirolo, A.; Al Arnaout, A.; Popescu, E.; Viart, G.; Godin, B.; Gillibert, A.; Savouré, A.; et al. Can permanent His bundle pacing be safely started by operators new to this technique? Data from a multicenter registry. J. Cardiovasc. Electrophysiol. 2021, 32, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Oren, J.W.; et al. His-SYNC Investigators. On-Treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: A secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm. 2019, 16, 1797–1807. [Google Scholar] [CrossRef]
- Vinther, M.; Risum, N.; Svendsen, J.H.; Møgelvang, R.; Philbert, B.T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients with Left Bundle Branch Block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef]
- Nagarajan, V.D.; Ho, S.Y.; Ernst, S. Anatomical Considerations for His Bundle Pacing. Circ. Arrhythm. Electrophysiol. 2019, 12, e006897. [Google Scholar] [CrossRef]
- Zheng, R.; Dong, Y.; Wu, S.; Su, L.; Zhao, D.; Chen, X.; Cai, B.; Fang, X.; Vijayaraman, P.; Huang, W. Conduction system pacing following septal myectomy: Insights into site of conduction block. J. Cardiovasc. Electrophysiol. 2022, 33, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.A.; Cherian, T.; Shatz, D.Y.; Beaser, A.D.; Aziz, Z.; Ozcan, C.; Broman, M.T.; Nayak, H.M.; Tung, R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation 2019, 139, 1876–1888. [Google Scholar] [CrossRef]
- Keene, D.; Arnold, A.D.; Jastrzębski, M.; Burri, H.; Zweibel, S.; Crespo, E.; Chandrasekaran, B.; Bassi, S.; Joghetaei, N.; Swift, M.; et al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J. Cardiovasc. Electrophysiol. 2019, 30, 1984–1993. [Google Scholar] [CrossRef]
- Zanon, F.; Abdelrahman, M.; Marcantoni, L.; Naperkowski, A.; Subzposh, F.A.; Pastore, G.; Baracca, E.; Boaretto, G.; Raffagnato, P.; Tiribello, A.; et al. Long term performance and safety of His bundle pacing: A multicenter experience. J. Cardiovasc. Electrophysiol. 2019, 30, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Miglioranza, M.H.; Mihăilă, S.; Peluso, D.; Xhaxho, J.; Marra, M.P.; Cucchini, U.; Soriani, N.; Iliceto, S.; Muraru, D. Left Atrial Volumes and Function by Three-Dimensional Echocardiography: Reference Values, Accuracy, Reproducibility, and Comparison with Two-Dimensional Echocardiographic Measurements. Circ. Cardiovasc. Imaging 2016, 9, e004229. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Ellenbogen, K.A. Approach to permanent His bundle pacing in challenging implants. Heart Rhythm. 2018, 15, 1428–1431. [Google Scholar] [CrossRef]
| N | 72 |
|---|---|
| Age (years, median (Q1–Q3)) | 70 (65–77) |
| Male sex | 53 (73.6%) |
| Height (cm, median (Q1–Q3)) | 172 (165–176.75) |
| Weight (kg, median (Q1–Q3)) | 80 (73.25–90) |
| BMI (kg/cm2, median (Q1–Q3)) | 27,72 (25.17–32.43) |
| Pacing indication | |
| Sick sinus node disease | 3 (4.2%) |
| Atrioventricular block | 45 (62.4%) |
| Resynchronization therapy | 11 (15.3%) |
| Pace and ablate | 12 (16.7%) |
| Pacing-induced cardiomyopathy | 1 (1.4%) |
| QRS duration (msec, median (Q1–Q3)) | 131 (100–160) |
| Normal QRS | 33 (45.8%) |
| Left bundle branch block | 23 (31.9%) |
| Right bundle branch block | 16 (22.2%) |
| Comorbidities | |
| Atrial fibrillation | 29 (40.3%) |
| Stroke | 6 (8.3%) |
| Diabetes mellitus | 21 (29.2%) |
| Hypertension | 57 (79.2%) |
| Ischemic cardiomyopathy | 22 (30.6%) |
| Renal insufficiency | 18 (25%) |
| Obstructive lung disease | 5 (6.9%) |
| Medical treatment | |
| Beta-blockers | 56 (77.8%) |
| Angiotensin-converting enzyme inhibitors | 56 (77.8%) |
| Mineralocorticoid receptor antagonists | 29 (40.3%) |
| ARNI | 9 (12.5%) |
| iSGLT2 | 2 (2.8%) |
| Anticoagulants | 31 (43%) |
| HBE Identification | Procedural Success | |||||
|---|---|---|---|---|---|---|
| YES | NO | p-Value | YES | NO | p-Value | |
| N | 59 | 13 | 33 | 39 | ||
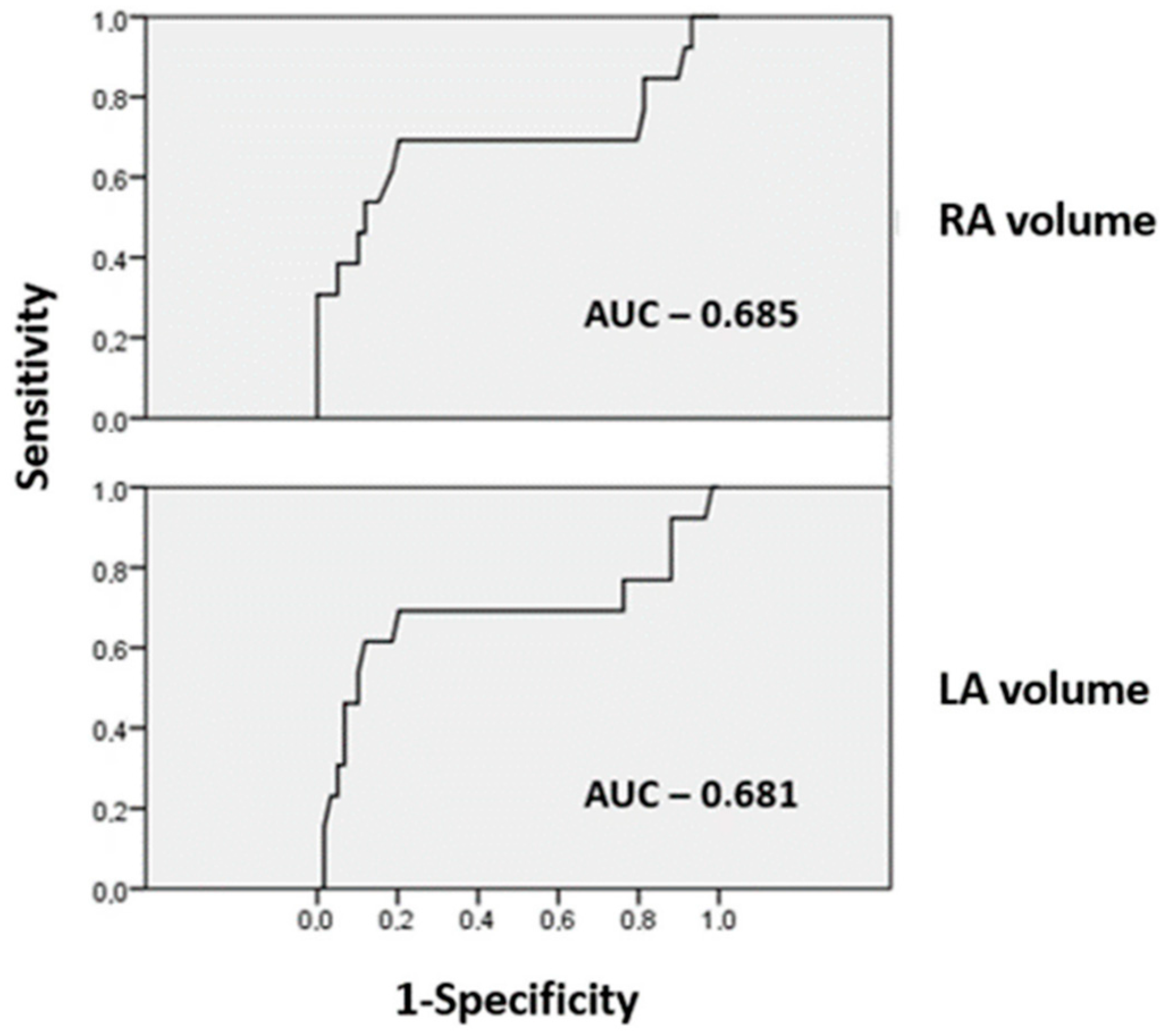
| LA volume (mL, mean ± SD) | 73.5 ± 33.1 | 106.3 ± 47.2 | 0.043 | 69.8 ± 23.3 | 87.6 ± 45.5 | 0.218 |
| RA volume (mL, mean ± SD) | 48.2 ± 17 | 69.2 ± 32.7 | 0.038 | 49.8 ± 18.1 | 53.8 ± 24.9 | 0.635 |
| LVES volume (mL, mean ± SD) | 69.6 ± 46.2 | 70.7 ± 34.4 | 0.578 | 65.1 ± 41 | 73.7 ± 46.7 | 0.403 |
| LVED volume (mL, mean ± SD) | 122.9 ± 51.9 | 122.7 ± 43 | 0.977 | 116.4 ± 42 | 128.3 ± 56.1 | 0.429 |
| RV basal diameter (mm, mean ± SD) | 31.4 ± 4.5 | 33.5 ± 3.6 | 0.115 | 30.7 ± 4.8 | 32.7 ± 3.7 | 0.062 |
| Ejection fraction (%, mean ± SD) | 48.8 ± 16.2 | 42.9 ± 14.3 | 0.139 | 49.7 ± 15.5 | 46.1 ± 16.2 | 0.222 |
| TAPSE (mm, mean ± SD) | 20.4 ± 4.6 | 17.6 ± 5.8 | 0.063 | 19.7 ± 4.9 | 20 ± 4.9 | 0.928 |
| Mitral regurgitation (mean ± SD) | 2.1 ± 0.8 | 2.4 ± 0.9 | 0.214 | 2.2 ± 0.8 | 2.1 ± 0.9 | 0.785 |
| Tricuspid regurgitation (mean ± SD) | 1.7 ± 0.8 | 1.9 ± 0.9 | 0.454 | 1.8 ± 0.8 | 1.7 ± 0.7 | 0.694 |
| HBE Identification Failure | Overall Procedural Failure | |||||
|---|---|---|---|---|---|---|
| OR | 95% C.I. | p-Value | OR | 95% C.I. | p-Value | |
| LA volume (mL) | 1.021 | 1.005–1.037 | 0.010 | 1.013 | 0.998–1.029 | 0.083 |
| RA volume (mL) | 1.047 | 1.013–1.082 | 0.006 | 1.005 | 0.982–1.028 | 0.689 |
| LVES volume (mL) | 1.002 | 0.987–1.016 | 0.813 | 1.003 | 0.991–1.015 | 0.629 |
| LVED volume (mL) | 1.001 | 0.987–1.014 | 0.914 | 1.003 | 0.992–1.014 | 0.603 |
| RV basal diameter (mm) | 1.168 | 0.985–1386 | 0.075 | 1.106 | 0.983–1.244 | 0.093 |
| Ejection fraction (%) | 0.975 | 0.939–1.012 | 0.184 | 0.985 | 0.955–1.016 | 0.336 |
| TAPSE (mm) | 0.891 | 0.783–1.014 | 0.081 | 1.010 | 0.917–1.112 | 0.840 |
| Mitral regurgitation | 1.523 | 0.746–3.109 | 0.248 | 0.985 | 0.562–1.728 | 0.959 |
| Tricuspid regurgitation | 1.321 | 0.603–2.894 | 0.487 | 0.910 | 0.488–1.696 | 0.766 |
| Height (cm) | 0.965 | 0.884–1.054 | 0.428 | 1.039 | 0.971–1.112 | 0.265 |
| Weight (kg) | 1.033 | 0.984–1.084 | 0.195 | 1.011 | 0.973–1.050 | 0.585 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestrea, C.; Cicala, E.; Ivascu, M.; Gherghina, A.; Pintilie, I.; Ortan, F.; Pop, D. The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—A Single Center Experience. J. Clin. Med. 2022, 11, 7076. https://doi.org/10.3390/jcm11237076
Pestrea C, Cicala E, Ivascu M, Gherghina A, Pintilie I, Ortan F, Pop D. The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—A Single Center Experience. Journal of Clinical Medicine. 2022; 11(23):7076. https://doi.org/10.3390/jcm11237076
Chicago/Turabian StylePestrea, Catalin, Ecaterina Cicala, Madalina Ivascu, Alexandra Gherghina, Irina Pintilie, Florin Ortan, and Dana Pop. 2022. "The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—A Single Center Experience" Journal of Clinical Medicine 11, no. 23: 7076. https://doi.org/10.3390/jcm11237076
APA StylePestrea, C., Cicala, E., Ivascu, M., Gherghina, A., Pintilie, I., Ortan, F., & Pop, D. (2022). The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—A Single Center Experience. Journal of Clinical Medicine, 11(23), 7076. https://doi.org/10.3390/jcm11237076





