The Nuts and Bolts of Implementing a Modified ERAS Protocol for Minimally Invasive Colorectal Surgery: Group Practice vs. Solo Practice
Abstract
1. What Does This Paper Add to the Literature?
2. Introduction
3. Methods
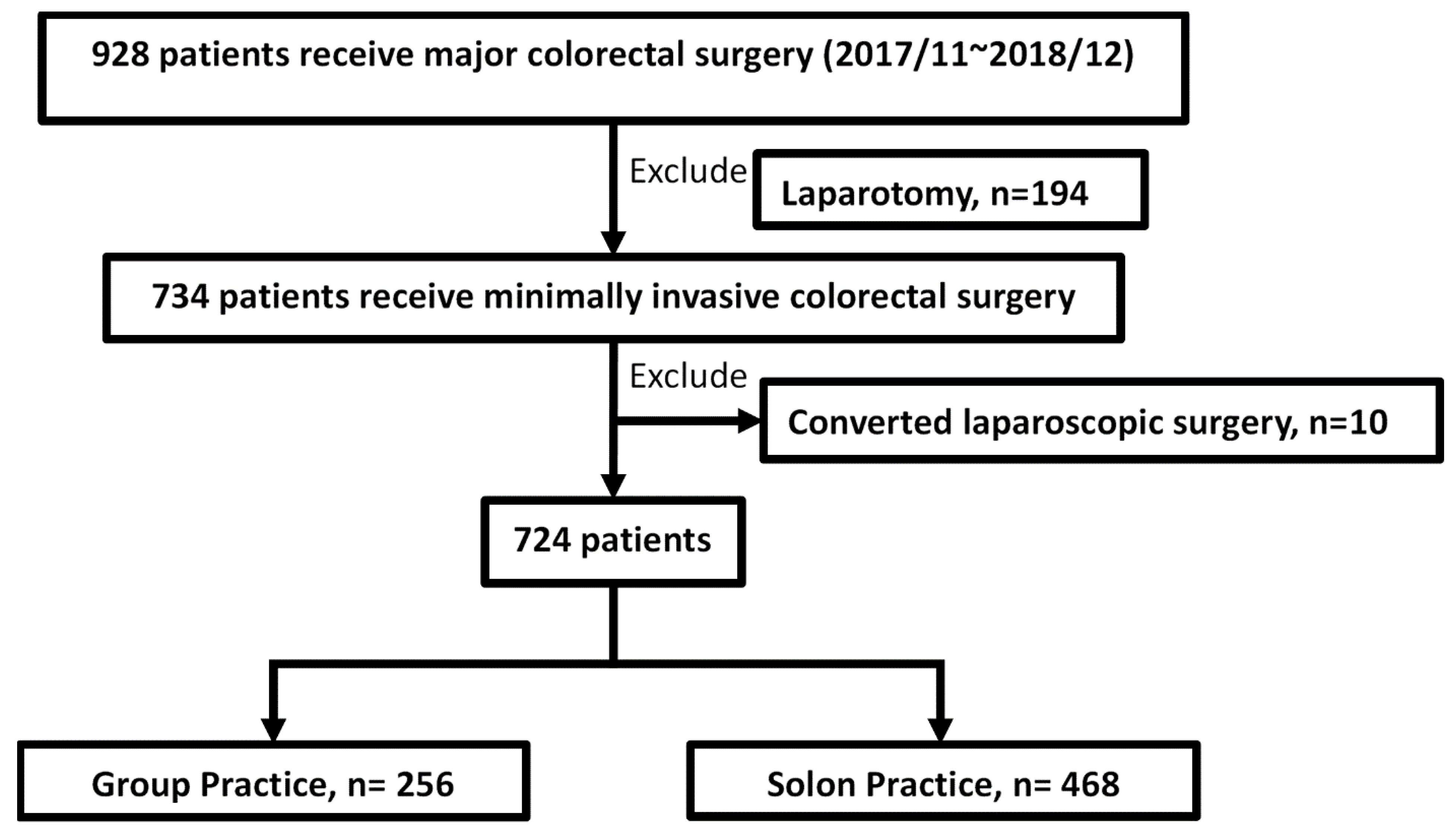
3.1. Study Design and Patient Selection
3.2. Solo Practice and Group Practice
3.3. The Modified ERAS Protocol
3.4. Outcomes and Covariables
3.5. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American society of colon and rectal surgeons and society of American gastrointestinal and endoscopic surgeons. Dis. Colon. Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery enhanced recovery after surgery (ERAS®) society recommendations 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, P.; Bordeianou, L. Implementation of an ERAS pathway in colorectal surgery. Clin. Colon. Rectal. Surg. 2019, 32, 102–108. [Google Scholar]
- Ban, K.A.; Berian, J.R.; Ko, C.Y. Does implementation of enhanced recovery after surgery (ERAS) protocols in colorectal surgery improve patient outcomes? Clin. Colon. Rectal. Surg. 2019, 32, 109–113. [Google Scholar]
- Nygren, J. The metabolic effects of fasting and surgery. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 429–438. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Oppelstrup, H.; Thorell, A.; Nygren, J.; Ljungqvist, O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery a retrospective cohort study. World J. Surg. 2016, 40, 1741–1747. [Google Scholar] [CrossRef]
- Miller, T.E.; Thacker, J.K.; White, W.D.; Mantyh, C.; Migaly, J.; Jin, J.; Roche, A.M.; Eisenstein, E.L.; Edwards, R.; Anstrom, K.J.; et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth. Analg. 2014, 118, 1052–1061. [Google Scholar] [CrossRef]
- Tan, S.Y.; Furubayashi, J.K. The Mayo brothers (William James Mayo 1861–1939, Charles Horace Mayo 1865–1939) pioneers of group surgical practice. Singap. Med. J. 2012, 53, 157–158. [Google Scholar]
- Paulick, J.M.; Roos, N.P. The young physician: Types of practice. Can. Med. Assoc. J. 1978, 118, 276–278. [Google Scholar] [PubMed]
- Casalino, L.P.; Devers, K.J.; Lake, T.K.; Reed, M.; Stoddard, J.J. Benefits of and barriers to large medical group practice in the United States. Arch. Intern. Med. 2003, 163, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Zwiep, T.; Ahn, S.H.; Brehaut, J.; Balaa, F.; McIsaac, D.I.; Rich, S.; Wallace, T.; Moloo, H. Group practice impacts on patients, physicians and healthcare systems a scoping review. BMJ Open 2021, 11, e041579. [Google Scholar] [CrossRef] [PubMed]
- Sigsbee, B. Physician compensation approach and models in neurological practice. Neurol. Clin. 2010, 28, 339–348. [Google Scholar] [CrossRef]
- Burns, L.A. Physicians and group practice balancing autonomy with market reality. J. Ambul. Care Manag. 1996, 19, 1–15. [Google Scholar]
- Pedziwiatr, M.; Mavrikis, J.; Witowski, J.; Adamos, A.; Major, P.; Nowakowski, M.; Budzyński, A. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med. Oncol. 2018, 35, 95. [Google Scholar] [CrossRef]
- Landon, B.E.; Normand, S.L.; Frank, R.; McNeil, B.J. Characteristics of medical practices in three developed managed care markets. Health Serv. Res. 2005, 40, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Fye, W.B. Presidential address: The origins and evolution of the Mayo Clinic from 1864 to 1939 a Minnesota family practice becomes an international “medical Mecca”. Bull. Hist. Med. 2010, 84, 323–357. [Google Scholar] [PubMed]
- Muhlestein, D.B.; Smith, N.J. Physician consolidation rapid movement from small to large group practices, 2013–15. Health Aff. (Millwood) 2016, 35, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.R.; Goldsmith, J.C.; Sen, A. Horizontal and vertical integration of physicians: A tale of two tails. Annu. Rev. Health Care Manag. 2013, 15, 39–117. [Google Scholar]
- Liebhaber, A.; Grossman, J.M. Physicians moving to mid-sized, single-specialty practices. Track Rep. 2007, 18, 1–5. [Google Scholar]
- Casalino, L.P.; Pham, H.; Bazzoli, G. Growth of single-specialty medical groups. Health Aff. (Millwood) 2004, 23, 82–90. [Google Scholar] [CrossRef]
- Rothenberger, D.A. Physician burnout and well-being: A systematic review and framework for action. Dis. Colon. Rectum 2017, 60, 567–576. [Google Scholar] [CrossRef]
- West, C.P.; Dyrbye, L.N.; Erwin, P.J.; Shanafelt, T.D. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet 2016, 388, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Hasan, O.; Dyrbye, L.N.; Sinsky, C.; Satele, D.; Sloan, J.; West, C.P. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin. Proc. 2015, 90, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H. Enhanced recovery after surgery (ERAS) good for now, but what about the future? Can. J. Anaesth. 2015, 62, 99–104. [Google Scholar] [CrossRef]
- Moiniche, S.; Dahl, J.B.; Rosenberg, J.; Kehlet, H. Colonic resection with early discharge after combined subarachnoid-epidural analgesia, preoperative glucocorticoids, and early postoperative mobilization and feeding in a pulmonary high-risk patient. Reg. Anesth. Pain Med. 1994, 19, 352–356. [Google Scholar]
- Kehlet, H. Organizing postoperative accelerated recovery programs. Reg. Anesth. Pain Med. 1996, 21, 149–151. [Google Scholar]
- Bardram, L.; Funch-Jensen, P.; Jensen, P.; Crawford, M.E.; Kehlet, H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995, 345, 763–764. [Google Scholar] [CrossRef]
- Bhardwaj, N. Enhanced recovery after surgery. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S3–S4. [Google Scholar] [CrossRef]
- Brindle, M.; Nelson, G.; Lobo, D.N.; Ljungqvist, O.; Gustafsson, U.O. Recommendations from the ERAS(R) Society for standards for the development of enhanced recovery after surgery guidelines. BJS Open 2020, 4, 157–163. [Google Scholar] [CrossRef]
- Brady, M.; Kinn, S.; Stuart, P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, 4, CD004423. [Google Scholar] [CrossRef]
- Nygren, J.; Thorell, A.; Ljungqvist, O. Preoperative oral carbohydrate nutrition: An update. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Biffi, R.; Sandini, M.; Marrelli, D.; Vignali, A.; Caccialanza, R.; Viganò, J.; Sabbatini, A.; Di Mare, G.; Alessiani, M.; et al. Preoperative oral carbohydrate load versus placebo in major elective abdominal surgery (PROCY) a randomized, placebo-controlled, multicenter, phase III trial. Ann. Surg. 2018, 267, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tweed, T.T.T.; Woortman, C.; Tummers, S.; Bakens, M.; van Bastelaar, J.; Stoot, J. Reducing hospital stay for colorectal surgery in ERAS setting by means of perioperative patient education of expected day of discharge. Int. J. Color. Dis. 2021, 36, 1535–1542. [Google Scholar] [CrossRef]
- Forsmo, H.M.; Pfeffer, F.; Rasdal, A.; Sintonen, H.; Korner, H.; Erichsen, C. Pre- and postoperative stoma education and guidance within an enhanced recovery after surgery (ERAS) programme reduces length of hospital stay in colorectal surgery. Int. J. Surg. 2016, 36, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.C.; Bao, X.; Agarwala, A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clin. Colon. Rectal. Surg. 2019, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Kehl, H. Postoperative pain management in the era of ERAS: An overview. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 259–267. [Google Scholar] [CrossRef]
- EuroSurg, C. Intraperitoneal drain placement and outcomes after elective colorectal surgery international matched, prospective, cohort study. Br. J. Surg. 2022, 109, 520–529. [Google Scholar] [CrossRef]
- Izquierdo, K.M.; Unal, E.; Marks, J.H. Natural orifice specimen extraction in colorectal surgery patient selection and perspectives. Clin. Exp. Gastroenterol. 2018, 11, 265–279. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chen, W.T. Natural orifice specimen extraction (NOSE) with single-stapling anastomosis for left colon cancer. J. Minim. Invasive Surg. 2020, 23, 201–203. [Google Scholar] [CrossRef]
- Chin, Y.H.; Decruz, G.M.; Ng, C.H.; Tan, H.Q.M.; Lim, F.; Foo, F.J.; Tai, C.H.; Chong, C.H. Colorectal resection via natural orifice specimen extraction versus conventional laparoscopic extraction a meta-analysis with meta-regression. Tech. Coloproctol. 2021, 25, 35–48. [Google Scholar] [CrossRef]
- Tavernier, C.; Flaris, A.N.; Passot, G.; Glehen, O.; Kepenekian, V.; Cotte, E. Assessing criteria for a safe early discharge after laparoscopic colorectal surgery. JAMA Surg. 2022, 157, 52–58. [Google Scholar] [CrossRef] [PubMed]
| Primary Component | Standard | Modified |
|---|---|---|
| Preadmission | ||
| Preadmission counselling | Information about preoperative education, surgical indication, and discussion of milestones and discharge criteria | The same |
| Preadmission optimization | Prehabilitation | The same |
| Preoperative interventions | ||
| Preoperative nutrition | Drink clear fluids continuously <2 h before the induction of anaesthesia Carbohydrate loading should be encouraged before surgery in nondiabetic patients | NPO at least 8 h before surgery Nutritionist referral and parenteral nutrition supplement if needed |
| Management of anaemia | Not mentioned | Blood transfusion to keep hemoglobin (Hb) > 10 gm/dL |
| Mechanical bowel preparation | Recommended | Commonly used except for nearly obstructive tumours and elderly patients |
| Oral antibiotic preparation | Recommended | Not routine |
| Perioperative interventions | ||
| Reduce surgical site infection | Preparation of surgical field with chlorhexidine Prophylactic antibiotics before incision | 2% chlorhexidine as antiseptic Cefazolin 1 gm + metronidazole 500 mg within 30 min of incision |
| Prevention of nausea and vomiting | Combination of ondansetron with dexamethasone before anaesthesia | Ondansetron before anaesthesia |
| Intraoperative fluid management | Avoid volume overload Balanced chloride-restricted crystalloid solutions should be used as maintenance Goal-directed fluid therapy | The same |
| Pain control | A multimodal, opioid-sparing, pain management plan before the induction of anaesthesia Transversus abdominis plane block with a local anaesthetic Thoracic epidural analgesia is recommended for open colorectal surgery, but not for routine use in laparoscopic colorectal surgery | Local anaesthetic at the end of surgery Multimodal pain control |
| Surgical approach | A minimally invasive surgical approach should be used | All minimally invasive surgery included in this study |
| Use of intra-abdominal drains and nasogastric (NG) tubes | Should not be routinely used | Remove NG tubes at the end of surgery Jackson-Pratt drain is optional |
| Postoperative interventions | ||
| Patient mobilization | Early and progressive patient mobilization | As soon as possible but not compulsive |
| Ileus prevention | A regular diet immediately after elective colorectal surgery | Progress gradually from clear liquid diet to full liquid diet and then soft diet according to patient’s condition and physician’s decision |
| Postoperative fluid management | Intravenous fluids should be discontinued in the early postoperative period | Discontinued if patient intake is smooth |
| Urinary catheters | Urinary catheters should be removed within 24 h of elective colonic or upper rectal resection when not involving a vesicular fistula. Urinary catheters should be removed within 48 h of mid/lower rectal resections. | Removed after patient mobilization under the same conditions. |
| Variables | Group Practices n = 256 | Solo Practices n = 468 | p |
|---|---|---|---|
| Operative procedure | 0.474 | ||
| Right hemicolectomy | 62 (24.2%) | 102 (21.8%) | |
| Left hemicolectomy | 22 (8.6%) | 35 (7.5%) | |
| Anterior resection | 88 (34.4%) | 159 (34.0%) | |
| Low anterior resection | 54 (21.1%) | 126 (26.9%) | |
| Others | 30 (11.7%) | 46 (9.8%) | |
| Combined surgery | 18 | 24 | 0.320 |
| Hepatectomy | 4 | 12 | 0.441 |
| Urology | 5 | 5 | 0.336 |
| Gynaecology | 9 | 7 | 0.110 |
| Blood loss < 50 mL | 195 (76.2%) | 357 (76.3%) | 0.400 |
| Surgical time (hh:mm) | 4:09 ± 1:33 | 4:13 ± 1:30 | 0.539 |
| Diagnosis | 0.202 | ||
| Malignancy | 218 (85.2%) | 414 (88.5%) | |
| Benign neoplasm | 9 (3.5%) | 15 (3.2%) | |
| Diverticular disease | 7 (2.7%) | 7 (1.5%) | |
| Constipation | 7 (2.7%) | 19 (4.0%) | |
| Others | 15 (5.9%) | 13 (2.8%) | |
| Cancer stage | 0.282 | ||
| Stage 0/I/II | 109 (50%) | 224 (54.1%) | |
| Stage III/IV | 109 (50%) | 190 (45.9%) | |
| NOSE | 76 (29.7%) | 107 (22.9%) | 0.043 |
| Operative method | <0.05 | ||
| Laparoscopy | 233 (91.0%) | 466 (99.6%) | |
| Robotic-assisted | 23 (9.0%) | 2 (0.4%) | |
| Diverting stoma | 24 (9.4%) | 63 (13.5) | 0.106 |
| Morbidity | 36 (14%) | 100 (21.4%) | 0.048 |
| Grade II | 28 (10.9%) | 73 (15.6%) | |
| Grade III | 8 (3.1%) | 27 (5.8%) | 0.113 |
| Readmission | 7 (2.7%) | 16 (3.4%) | 0.616 |
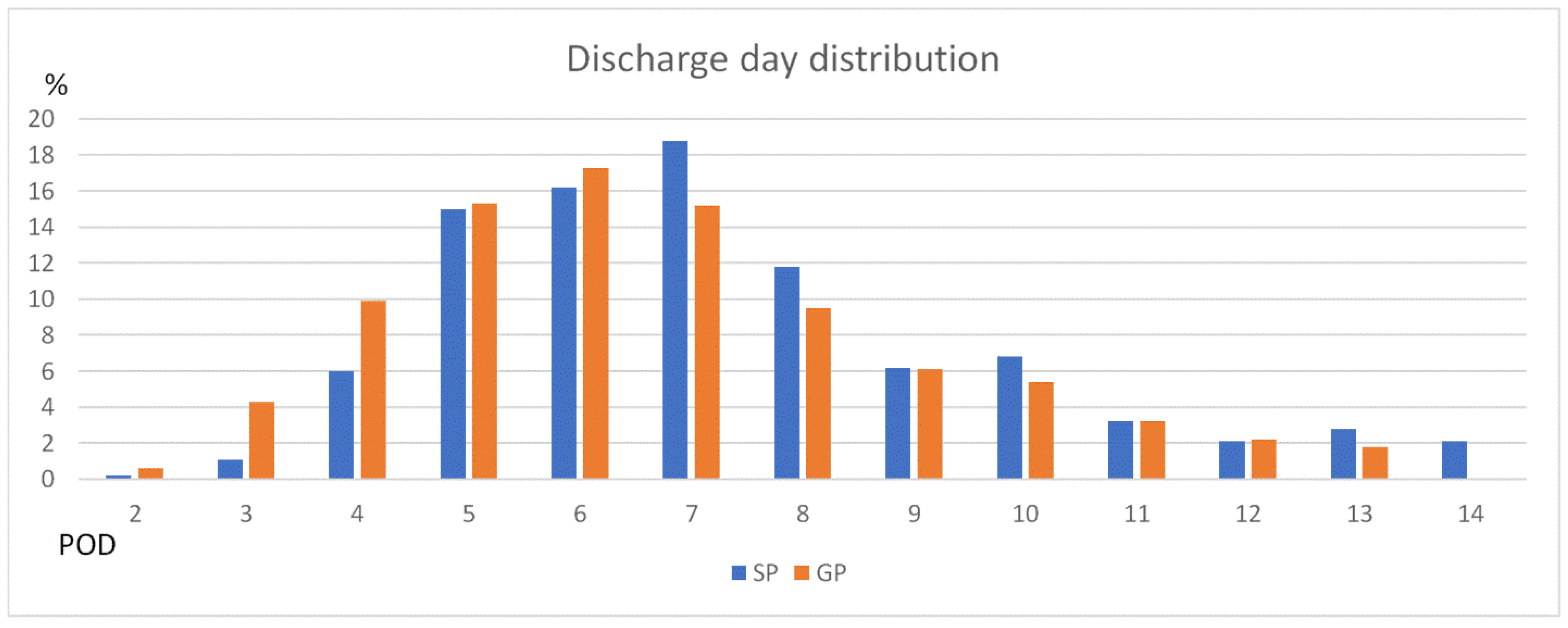
| Discharge day | |||
| ≤4 days | 73 (28.5%) | 33 (7.1%) | <0.05 |
| ≤5 days | 114 (44.5%) | 104 (22.2%) | <0.05 |
| ≤6 days | 162 (63.3%) | 179 (38.2%) | <0.05 |
| Discharge day (POD) | |||
| Mean | 6.6 ± 3.2 | 8.6 ± 5.5 | <0.05 |
| Median | 6 | 7 | |
| Postoperative blood test | |||
| WBC ≥ 104/dL | 103 (40.2%) | 158 (33.8%) | 0.13 |
| Hb ≥ 10 g/dL | 195 (76.2%) | 345 (73.7%) | 0.489 |
| CRP (mg/dL) | 80.91 ± 51.15 | 73.88 ± 62.24 | 0.108 |
| Pain score | 2.25 ± 0.77 | 2.36 ± 0.68 | 0.048 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | p | ||
| Group practice | 2.810 (2.022–3.905) | <0.001 | 2.836 (1.985–4.051) | <0.001 |
| NOSE | 3.790 (2.662–5.396) | <0.001 | 3.488 (2.333–5.096) | <0.001 |
| Operative method | <0.001 | 0.24 | ||
| Right hemicolectomy | REF | REF | ||
| Left hemicolectomy | 1.232 (0.651–2.331) | 0.521 | 1.161 (0.578–2.331) | 0.674 |
| Anterior resection | 1.450 (0.953–2.205) | 0.083 | 1.170 (0.736–1.859) | 0.507 |
| Low anterior resection | 0.610 (0.374–0.994) | 0.047 | 0.608 (0.354–1.043) | 0.071 |
| Others | 0.515 (0.264–1.005) | 0.052 | 0.471 (0.224–0.991) | 0.047 |
| Robotic surgery | 1.368 (0.580 × 3.226) | 0.474 | ||
| Male | 0.950 (0.690–1.307) | 0.751 | NS | |
| Age < 65 y | 1.109 (0.804–1.529) | 0.530 | NS | |
| BMI > 25 kg/m2 | 1.313 (0.952–1.811) | 0.096 | NS | |
| Neoadjuvant therapy | 0.445 (0.228–0.868) | 0.018 | 0.721 (0.336–1.547) | 0.401 |
| ASA 3 (ref ASA 2) | 0.809 (0.578–1.131) | 0.215 | NS | |
| Tumour > 4 cm | 0.675 (0.481–0.949) | 0.024 | 0.929 (0.614–1.404) | 0.726 |
| Stage | 0.407 | NS | ||
| Benign disease | REF | 0.818 | ||
| Early stage I/II | 0.944 (0.579–1.539) | 0.300 | ||
| Advanced stage III/IV | 0.768 (0.466–1.265) | |||
| Blood loss > 50 mL | 0.388 (0.214–0.703) | 0.002 | 0.504 (0.263–0.965) | 0.039 |
| Preop WBC >10 k | 1.179 (0.696–1.999) | 0.540 | NS | |
| Preop Hb > 10 | 1.239 (0.762–2.017) | 0.388 | NS | |
| Preop Alb >3.5 | 2.071 (0.948–4.524) | 0.068 | 1.873 (0.788–4.448) | 0.155 |
| Preop CEA > 5 | 0.503 (0.332–0.762) | 0.001 | 0.654 (0.413–1.037) | 0.071 |
| Preop CRP > 5 | 0.593 (0.398–0.883) | 0.010 | 0.834 (0.519–1.341) | 0.454 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.-H.; Chern, Y.-J.; Hsu, Y.-J.; Jong, B.-K.; Tsai, W.-S.; Hsieh, P.-S.; Cheng, C.-C.; You, J.-F. The Nuts and Bolts of Implementing a Modified ERAS Protocol for Minimally Invasive Colorectal Surgery: Group Practice vs. Solo Practice. J. Clin. Med. 2022, 11, 6992. https://doi.org/10.3390/jcm11236992
Yu Z-H, Chern Y-J, Hsu Y-J, Jong B-K, Tsai W-S, Hsieh P-S, Cheng C-C, You J-F. The Nuts and Bolts of Implementing a Modified ERAS Protocol for Minimally Invasive Colorectal Surgery: Group Practice vs. Solo Practice. Journal of Clinical Medicine. 2022; 11(23):6992. https://doi.org/10.3390/jcm11236992
Chicago/Turabian StyleYu, Zhen-Hao, Yih-Jong Chern, Yu-Jen Hsu, Bor-Kang Jong, Wen-Sy Tsai, Pao-Shiu Hsieh, Ching-Chung Cheng, and Jeng-Fu You. 2022. "The Nuts and Bolts of Implementing a Modified ERAS Protocol for Minimally Invasive Colorectal Surgery: Group Practice vs. Solo Practice" Journal of Clinical Medicine 11, no. 23: 6992. https://doi.org/10.3390/jcm11236992
APA StyleYu, Z.-H., Chern, Y.-J., Hsu, Y.-J., Jong, B.-K., Tsai, W.-S., Hsieh, P.-S., Cheng, C.-C., & You, J.-F. (2022). The Nuts and Bolts of Implementing a Modified ERAS Protocol for Minimally Invasive Colorectal Surgery: Group Practice vs. Solo Practice. Journal of Clinical Medicine, 11(23), 6992. https://doi.org/10.3390/jcm11236992







