Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Subjects
2.3. Ultrasound-Guided Local Paricalcitol Injection (US-Guided LPI)
2.4. Intravenously Administered Paricalcitol
2.5. Laboratory Tests and Follow-Up
2.6. Human Parathyroid Gland Isolation and Ex Vivo Culture
2.7. Apoptosis Analysis
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Outcomes
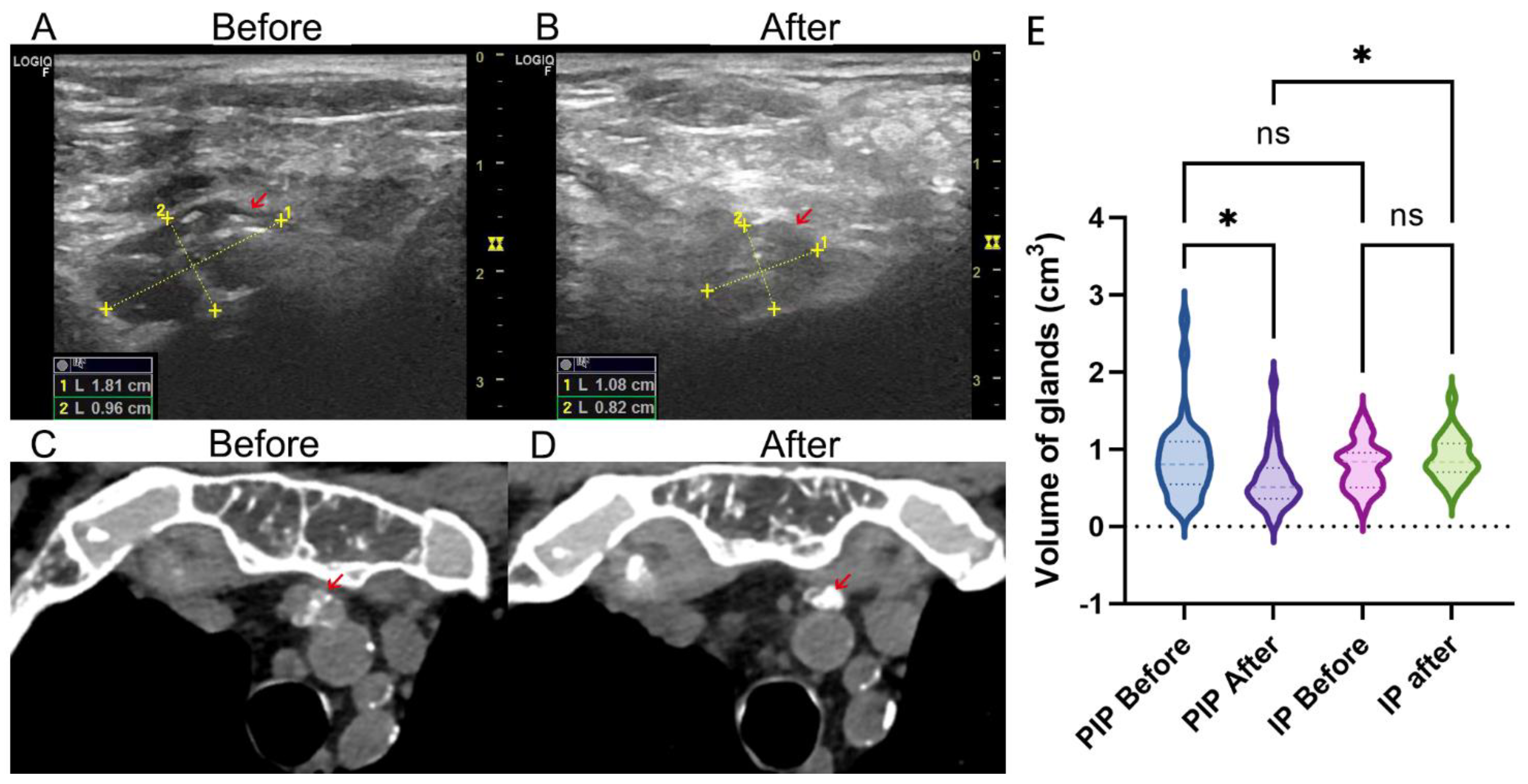
3.3. Volume Change of Parathyroid Gland after Therapy
3.4. Complications
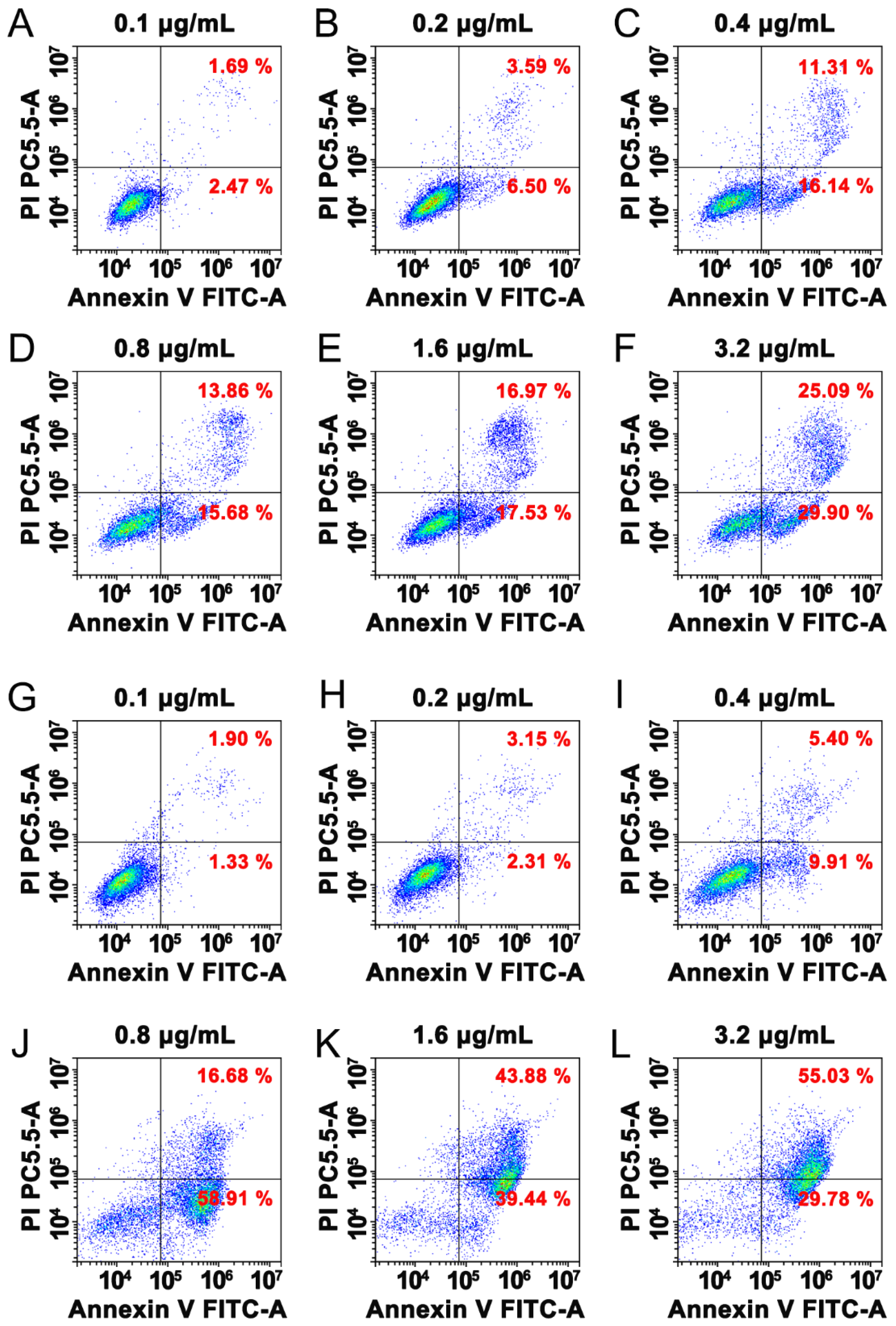
3.5. Paricalcitol Induced Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tentori, F.; Wang, M.; Bieber, B.A.; Karaboyas, A.; Li, Y.; Jacobson, S.H.; Andreucci, V.E.; Fukagawa, M.; Frimat, L.; Mendelssohn, D.C.; et al. Recent Changes in Therapeutic Approaches and Association with Outcomes among Patients with Secondary Hyperparathyroidism on Chronic Hemodialysis: The DOPPS Study. Clin. J. Am. Soc. Nephrol. 2015, 10, 98–109. [Google Scholar] [CrossRef]
- Centeno, P.P.; Herberger, A.; Mun, H.-C.; Tu, C.-L.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Fukagawa, M.; Hirakata, H.; Kagimura, T.; Fukushima, M.; Akizawa, T.; Suzuki, M.; Nishizawa, Y.; Yamazaki, C.; Tanaka, S.; et al. Effect of Treating Hyperphosphatemia with Lanthanum Carbonate vs Calcium Carbonate on Cardiovas-cular Events in Patients with Chronic Kidney Disease Undergoing Hemodialysis: The LANDMARK Randomized Clinical Trial. JAMA 2021, 325, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, P.S.; Chertow, G.M.; Block, G.A.; Correa-Rotter, R.; Drüeke, T.B.; Floege, J.; Herzog, C.A.; London, G.M.; Mahaffey, K.W.; Moe, S.M.; et al. The Clinical Course of Treated Hyperparathyroidism Among Patients Receiving Hemodialysis and the Effect of Cinacalcet: The EVOLVE Trial. J. Clin. Endocrinol. Metab. 2013, 98, 4834–4844. [Google Scholar] [CrossRef]
- Ivarsson, K.M.; Akaberi, S.; Isaksson, E.; Reihnér, E.; Rylance, R.; Prütz, K.-G.; Clyne, N.; Almquist, M. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2015, 30, 2027–2033. [Google Scholar] [CrossRef]
- Uludag, M.; Kartal, K. The Impact of Surgical Strategy on the Consequences of Secondary Hyperparathyroidism. Ann. Surg. 2018, 268, e62–e63. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, L.; Giangrande, A.; De Pra, L.; Bellotti, E.; Cantu, P.; Ravetto, C. Percutaneous ethanol injection of parathyroid tumors under US guidance: Treatment for secondary hyperparathyroidism. Radiology 1985, 155, 607–610. [Google Scholar] [CrossRef]
- Sung, J.Y.; Baek, J.H.; Kim, K.S.; Lee, D.; Yoo, H.; Kim, J.K.; Park, S.H. Single-Session Treatment of Benign Cystic Thyroid Nodules with Ethanol versus Radiofrequency Ablation: A Prospective Randomized Study. Radiology 2013, 269, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Hegedüs, L.; Pacella, C.M.; Baek, J.H.; Papini, E. Nonsurgical, Image-Guided, Minimally Invasive Therapy for Thyroid Nodules. J. Clin. Endocrinol. Metab. 2013, 98, 3949–3957. [Google Scholar] [CrossRef]
- Andress, D. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006, 69, 33–43. [Google Scholar] [CrossRef]
- Andress, D.L. Intravenous versus oral vitamin d therapy in dialysis patients: What is the question? Am. J. Kidney Dis. 2001, 38 (Suppl 5), S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; Blayney, M.J.; Albert, J.M.; Gillespie, B.W.; Kerr, P.G.; Bommer, J.; Young, E.W.; Akizawa, T.; Akiba, T.; Pisoni, R.L.; et al. Mortality Risk for Dialysis Patients with Different Levels of Serum Calcium, Phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2008, 52, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Naves-Diaz, M.; Passlick-Deetjen, J.; Guinsburg, A.; Marelli, C.; Fernandez-Martin, J.L.; Rodriguez-Puyol, D.; Cannata-Andia, J.B. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol. Dial. Transplant. 2011, 26, 1938–1947. [Google Scholar] [CrossRef]
- Robinson, D.M.; Scott, L.J. Paricalcitol: A review of its use in the management of secondary hyperparathyroidism. Drugs 2005, 65, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Ureña-Torres, P.; Lloret, M.J.; Ruiz, C.; DaSilva, I.; Diaz-Encarnacion, M.M.; Mercado, C.; Mateu, S.; Fernández, E.; Ballarin, J. Integral pharmacological management of bone mineral disorders in chronic kidney disease (part II): From treatment of phosphate imbalance to control of PTH and prevention of progression of cardiovascular calcification. Expert Opin. Pharmacother. 2016, 17, 1363–1373. [Google Scholar] [CrossRef]
- Kinnaert, P.; Salmon, I.; DeCoster-Gervy, C.; Vienne, A.; De Pauw, L.; Hooghe, L.; Tielemans, C. Long-term results of subcutaneous parathyroid grafts in uremic patients. Arch. Surg. 2000, 135, 186–190. [Google Scholar] [CrossRef]
- Schlosser, K.; Bartsch, D.K.; Diener, M.K.; Seiler, C.M.; Bruckner, T.; Nies, C.; Meyer, M.; Neudecker, J.; Goretzki, E.; Glockzin, G.; et al. Total Parathyroidectomy with Routine Thymectomy and Autotransplantation Versus Total Parathyroidectomy Alone for Secondary Hyperparathyroidism: Results of a Nonconfirmatory Multicenter Prospective Randomized Controlled Pilot Trial. Ann. Surg. 2016, 264, 745–753. [Google Scholar] [CrossRef]
- Singh Ospina, N.; Thompson, G.B.; Lee, R.A.; Reading, C.C.; Young, W.F., Jr. Safety and Efficacy of Percutaneous Parathyroid Ethanol Ablation in Patients with Recurrent Primary Hyperparathyroidism and Multiple Endocrine Neoplasia Type 1. J. Clin. Endocrinol. Metab. 2015, 100, E87–E90. [Google Scholar] [CrossRef]
- Yu, M.-A.; Yao, L.; Zhang, L.; Peng, L.; Zhuo, L.; Zhang, Y.; Li, W.; Lv, M.-D. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: A retrospective pilot study. Int. J. Hyperth. 2016, 32, 180–186. [Google Scholar] [CrossRef]
- Zeng, Z.; Peng, C.-Z.; Liu, J.-B.; Li, Y.-W.; He, H.-F.; Hu, Q.-H.; Lin, B.; Shen, X.-G. Efficacy of Ultrasound-guided Radiofrequency Ablation of Parathyroid Hyperplasia: Single Session vs. Two-Session for Effect on Hypocalcemia. Sci. Rep. 2020, 10, 6206. [Google Scholar] [CrossRef] [PubMed]
- Shiizaki, K.; Hatamura, I.; Negi, S.; Sakaguchi, T.; Saji, F.; Imazeki, I.; Kusano, E.; Shigematsu, T.; Akizawa, T. Highly concentrated calcitriol and its analogues induce apoptosis of parathyroid cells and regression of the hyperplastic gland—Study in rats. Nephrol. Dial. Transplant. 2008, 23, 1529–1536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhuo, L.; Peng, L.-L.; Zhang, Y.-M.; Xu, Z.-H.; Zou, G.-M.; Wang, X.; Li, W.-G.; Lu, M.-D.; Yu, M.-A. US-guided Microwave Ablation of Hyperplastic Parathyroid Glands: Safety and Efficacy in Patients with End-Stage Renal Disease—A Pilot Study. Radiology 2017, 282, 576–584. [Google Scholar] [CrossRef]
- Kovatcheva, R.D.; Vlahov, J.D.; Stoinov, J.I.; Zaletel, K. Benign Solid Thyroid Nodules: US-guided High-Intensity Focused Ultrasound Ablation—Initial Clinical Outcomes. Radiology 2015, 276, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
| LPI Group (n = 24) | IP Group (n = 22) | p | |
|---|---|---|---|
| Age, y | 46.07 ± 12.69 | 51.27 ± 12.97 | 0.18 |
| Sex, male, no. (%) | 14, 58.33% | 12, 54.55% | 0.99 |
| Primary disease type, no. (%) | 0.84 | ||
| Chronic nephritis | 10, 41.66% | 11, 50% | |
| Diabetes | 6, 25% | 5, 22.73% | |
| Hypetension | 8, 33.33% | 6, 27.27% | |
| Dialysis duration, mo | 86.25 ± 29.95 | 79.85 ± 23.21 | 0.43 |
| Hemoglobin | 112.93 ± 17.62 | 116.73 ± 11.15 | 0.39 |
| Preoperative medicine usage | 0.40 | ||
| Calcitriol | 8, 33.3% | 6, 27.27% | |
| Cinacalcet | 17, 70.83% | 5, 22.73% | |
| Phosphate binder | 19, 79.17% | 18, 81.82% |
| LPI Group | IP Group (n = 22) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Session 1 (n = 24) | Session 2 (n = 17) | Session 3 (n = 8) | End Follow Up (n = 24) | Before | After | ||||
| Before | After | Before | After | Before | After | ||||
| iPTH (pg/mL) | 1887.81 ± 726.81 | 1061.77 ± 701.49 * | 1625.81 ± 795.69 | 876.12 ±501.01 * | 1208.89 ± 322.06 | 831.32 ± 280.74 * | 631.06 ± 393.06 # | 686.87 ± 260.44 | 388.47 ± 167.36 # |
| Calcium (mg/dL) | 2.26 ± 0.22 | 2.16 ± 0.28 | 2.23 ± 0.29 | 2.15 ± 0.29 | 2.31 ± 0.08 | 2.22 ± 0.11 | 2.12 ± 0.23 | 2.36 ± 0.17 | 2.43 ± 0.11 |
| Phosphate (mg/dL) | 1.94 ± 0.36 | 1.86 ± 0.49 | 1.76 ± 0.41 | 1.84 ± 0.47 | 1.40 ± 0.42 | 1.48 ± 0.24 | 1.71 ± 0.34 # | 2.07 ± 0.40 | 2.06 ± 0.46 |
| Clinial Symptoms | IP Group (n = 22) | LPI Group (n = 24) | ||
|---|---|---|---|---|
| Incident | Remission | Incident | Remission | |
| Ostealgia | 9, 40.9% | 1, 11.11% | 21, 87.5% ** | 19, 90.5% * |
| Arthralgia | 2, 9.09% | 0, 0% | 3, 12.5% | 2, 66.6% |
| Cutaneous pruritus | 2, 9.09% | 0, 0% | 2, 8.33% | 1, 50% |
| Symptom | LPI Group (n = 24) | IP Group (n = 22) | ||
|---|---|---|---|---|
| Number | Durition Time (Day) | Number | Durition Time (Day) | |
| Facial numbness (n, %) | 1, 4.17% | 1 | ||
| Hoarseness (n, %) | 3, 12.5% | 2.33 ± 0.57 | ||
| Cough (n, %) | 1, 4.17% | 1 | ||
| Conjunctivitis (n, %) | 2, 9.09% | 1 | ||
| Palpitation (n, %) | 1, 4.54% | 0.5 | ||
| Subcutaneous induration (n, %) | 1, 4.54% | 3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Yu, Y.; Liu, Y.; Zhang, S.; Yuan, S.; Fan, K.; Tang, B.; Zhou, Q.; Sun, Y.; Liu, R.; et al. Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study. J. Clin. Med. 2022, 11, 6860. https://doi.org/10.3390/jcm11226860
Xie S, Yu Y, Liu Y, Zhang S, Yuan S, Fan K, Tang B, Zhou Q, Sun Y, Liu R, et al. Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study. Journal of Clinical Medicine. 2022; 11(22):6860. https://doi.org/10.3390/jcm11226860
Chicago/Turabian StyleXie, Shuqin, Yuan Yu, Yi Liu, Siliang Zhang, Shiyi Yuan, Kui Fan, Bin Tang, Qin Zhou, Yuqing Sun, Rui Liu, and et al. 2022. "Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study" Journal of Clinical Medicine 11, no. 22: 6860. https://doi.org/10.3390/jcm11226860
APA StyleXie, S., Yu, Y., Liu, Y., Zhang, S., Yuan, S., Fan, K., Tang, B., Zhou, Q., Sun, Y., Liu, R., Cao, D., Chen, Y., Wang, Y., Liu, G., Ma, H., Tao, C., Zeng, L., & Zhong, L. (2022). Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study. Journal of Clinical Medicine, 11(22), 6860. https://doi.org/10.3390/jcm11226860




