Evaluation of the Tooth Surface after Irradiation with Diode Laser Applied for Removal of Dental Microorganisms from Teeth of Patients with Gingivitis, Using X-ray Photoelectron (XPS) and Optical Profilometry (OP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Materials
2.2. Methods
2.2.1. Assessment of Microbiological Contamination
2.2.2. Laser Irradiation
Laser Parameters
- L1—decontaminate implant: 1 W/CW, average = 1.0 W, tip 600 μm, t = 1 min
- L2—expose implant: 15 W/15.000 Hz/10 µs, average = 2.30 W, tip 600 µm, t = 1 min
- L3a—periimplantitis surgical: 25 W/15.000 Hz/10 µs, average = 3.84 W, tip 600 µm, t = 1 × 15 s,
- L3b—periimplantitis surgical: 25 W/15.000 Hz/10 µs, average = 3.84 W, tip 600 µm t = 2 × 15 s,
- L3c—periimplantitis surgical: 25 W/15.000 Hz/10 µs, average = 3.84 W, tip 600 µm t = 3 × 15 s with 1 min cooling interval to eliminate overheating.
Assessment of Decontamination Effectiveness on the Tooth Surface
2.2.3. Statistical Analysis
2.2.4. Surface Morphology Analysis
X-ray Photoelectron Spectroscopy
Optical Profilometry
3. Results
3.1. Quantitative and Qualitative Analysis of Microbial Contamination of Teeth Surfaces
3.2. Evaluation of the Biocidal Effect of Laser Irradiation
3.3. Surface Morphology Analysis
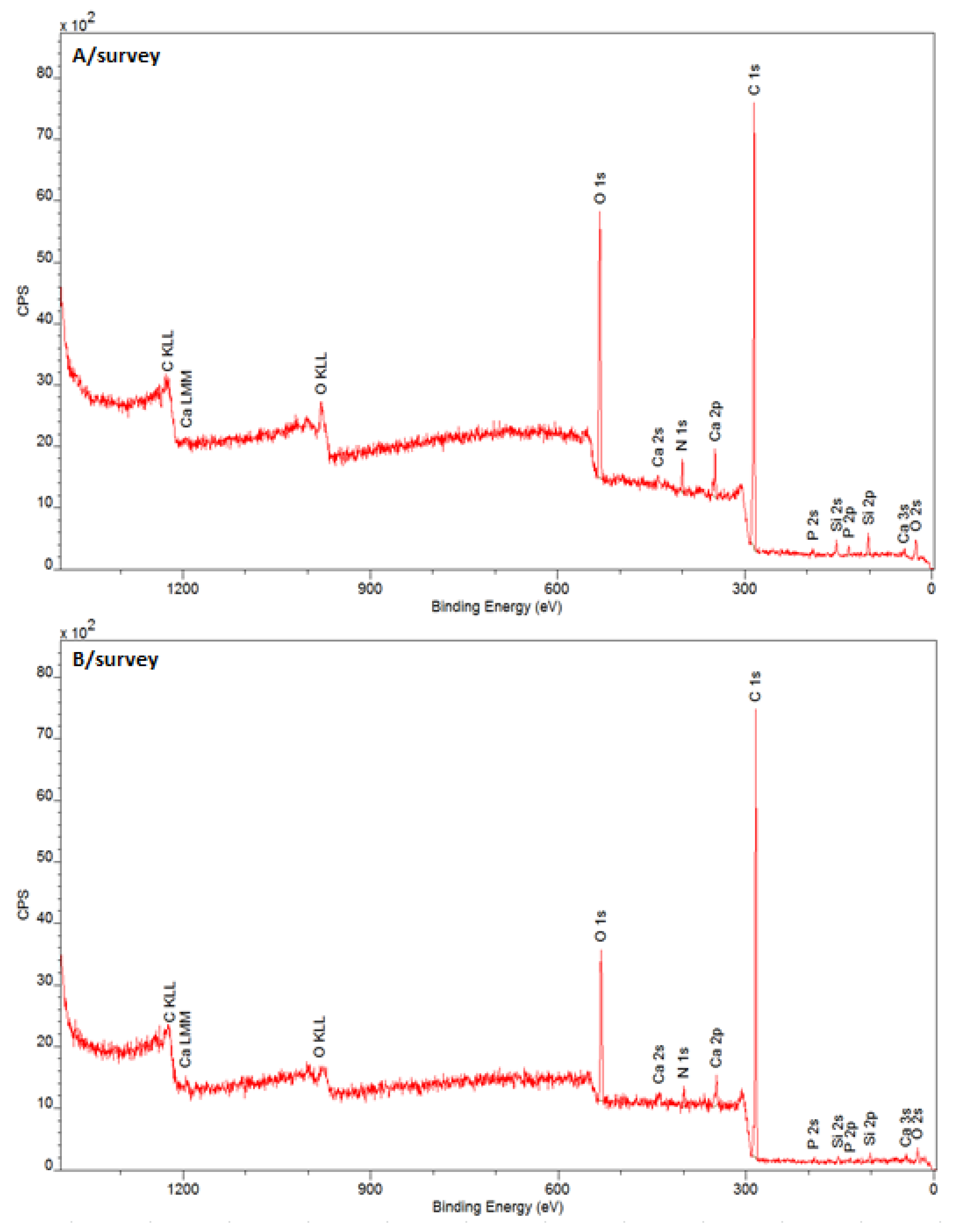
3.3.1. The Chemical Structure of the Tooth Surface before and after Laser Irradiation
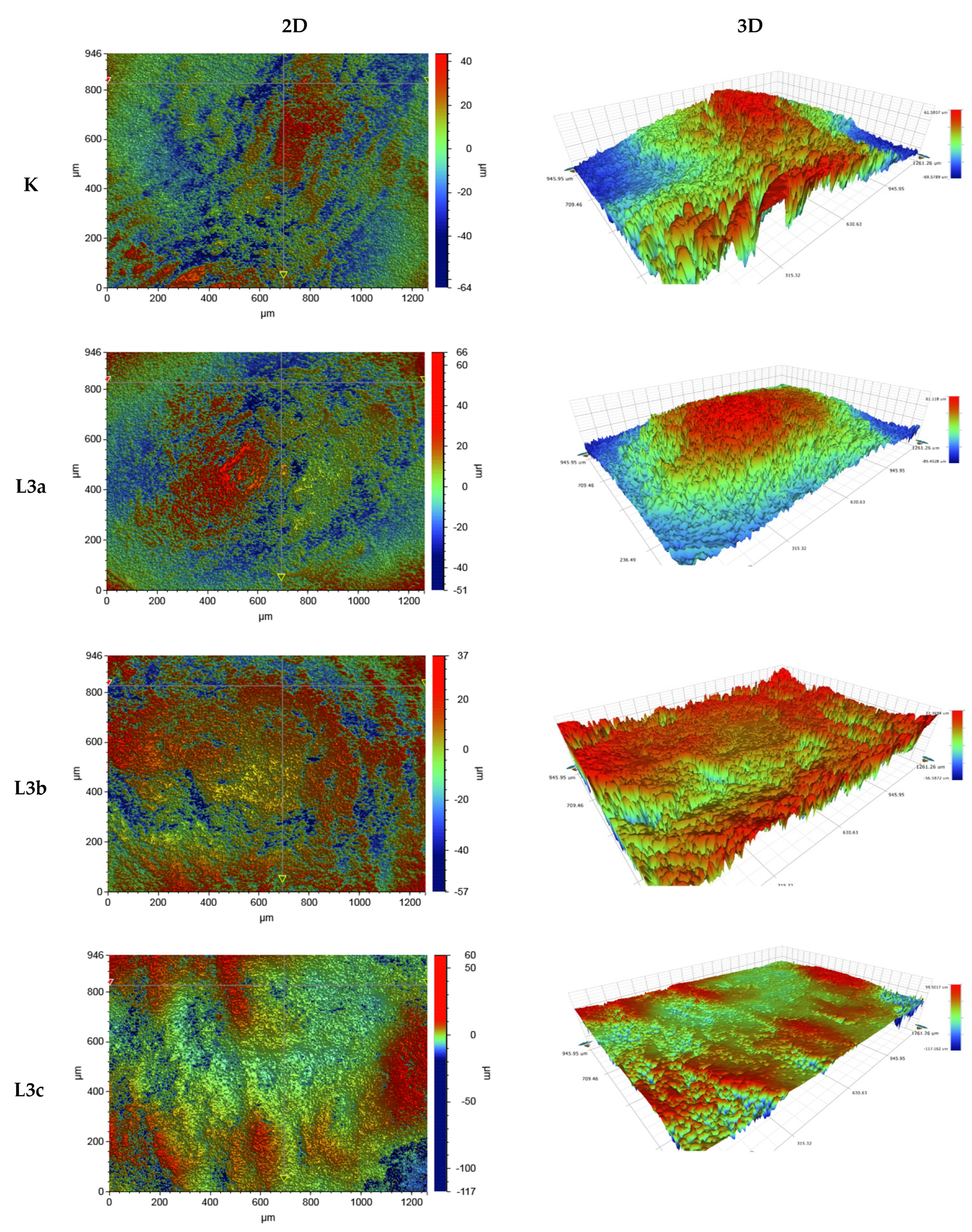
3.3.2. Roughness of the Tooth Surface before and after Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.S. Advancements toward a systems level understanding of the human oral microbiome. Front. Cell. Infect. Microbiol. 2014, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol. 2000 1997, 14, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Nele, V.; Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Panagakos, F.; Scannapieco, F. Periodontal Inflammation: From Gingivitis to Systemic Disease? In Gingival Diseases—Their Aetiology, Prevention and Treatment; Books on Demand: Norderstedt, Germany, 2011; pp. 154–168. [Google Scholar] [CrossRef]
- Tzanakakis, E.-G.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The Use of Lasers in Dental Materials: A Review. Materials 2021, 14, 3370. [Google Scholar] [CrossRef]
- Featherstone, J.; Nelson, D. Laser Effects on Dental Hard Tissues. Adv. Dent. Res. 1987, 1, 21–26. [Google Scholar] [CrossRef]
- Fried, D. IR laser ablation of dental enamel. Proceedings of SPIE. Int. Soc. Opt. Eng. 2000, 3910, 136–138. [Google Scholar] [CrossRef]
- Fried, D.; Glena, R.E.; Featherstone, J.D.; Seka, W. Permanent and transient changes in the reflectance of CO2 la-ser-irradiated dental hard tissues at lambda = 9.3, 9.6, 10.3, and 10.6 microns and at fluences of 1–20 J/cm2. Lasers Surg. Med. 1997, 20, 22–31. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Rybitwa, D.; Wilczyński, S.; Machoy, M.; Łobacz, M. Effective microbiological decontamination of dental healing abutments colonised with Rothia aeria by a diode laser as a helpful step towards successful implantoprosthetic therapy. Lasers Med. Sci. Res. 2020, 36, 875–887. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The efficacy of a diode laser on titanium implants for the reduction of microorganisms that cause periimplantitis. Materials 2021, 14, 7215. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Gutarowska, B.; Konka, A.; Zeljas, D.; Łobacz, M. Analysis of the Microbiome on the Surface of Corroded Titanium Dental Implants in Patients with Periimplantitis and Diode Laser Irradiation as an Aid in the Implant Prosthetic Treatment: An Ex Vivo Study. Materials 2022, 15, 5890. [Google Scholar] [CrossRef] [PubMed]
- Rybitwa, D.; Wawrzyk, A.; Łobacz, M.; Machoy, M.; Zeljaś, D.; Wilczyński, S. Hyperspectral imaging and directional reflectance for predicting interaction of laser radiation with biodeteriorated objects threatening human health. Int. Biodeterior. Biodegrad. 2022, 173, 105440. [Google Scholar] [CrossRef]
- Erdemir, U.; Sancakli, H.S.; Yildiz, E. The effect of one-step and multi-step polishing systems on the surface roughness and microhardness of novel resin composites. Eur. J. Dent. 2012, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bollenl, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Łobacz, M. The use of the diode laser against the microbiome on composites closing the screw access hall (SAH) in the reconstruction of dental implants. Ex Vivo studies. Int. J. Environ. Res. Public Health 2022, 19, 7494. [Google Scholar] [CrossRef]
- Taraboanta, I.; Stoleriu, S.; Nica, I.; Georgescu, A.; Gamen, A.C.; Maftei, G.A.; Andrian, S. Roughness variation of a nonhybrid composite resin submitted to acid and abrasive challenges. Int. J. Med. Dent. 2020, 24, 182–187. [Google Scholar] [CrossRef]
- Preoteasa, E.A.; Preoteasa, E.S.; Suciu, I.; Bartok, R.N.; Ltd., B.2.H.D.S. Atomic and nuclear surface analysis methods for dental materials: A review. AIMS Mater. Sci. 2018, 5, 781–844. [Google Scholar] [CrossRef]
- Stefański, T.; Malara, P.; Kloc-Ptaszna, A.; Postek-Stefańska, L. Przegląd Metod Badania Zmian Erozyjnych Zębów. DENTALFORUM/2/2015/XLIII. Available online: http://www.dentalforum.ump.edu.pl/uploads/2015/2/75_2_43_2015.pdf (accessed on 12 February 2015).
- Human Oral Microbiome Database. 2021. Available online: http://www.homd.org./ (accessed on 9 November 2021).
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Campos, T.M.B.; Marinho, R.M.D.M.; Ribeiro, A.D.O.P.; Montanheiro, T.L.D.A.; da Silva, A.C.; Thim, G.P. Microstructure and mechanical properties of fully sintered zirconia glazed with an experimental glass. J. Mech. Behav. Biomed. Mater. 2020, 113, 104093. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The Use of a Diode Laser for Removal of Microorganisms from the Surfaces of Zirconia and Porcelain Applied to Superstructure Dental Implants. Microorganisms 2021, 9, 2359. [Google Scholar] [CrossRef]
- Tonetto, M.R.; De Andrade, M.F.; Pinto, S.C.S.; Lima, D.M.; Saad, J.R.C.; Bandéca, M.C.; De Mendonça, A.A.M.; Elossais, A.A. Human Dental Enamel and Dentin Structural Effects after Er:yag Laser Irradiation. J. Contemp. Dent. Pract. 2014, 15, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, A.; Kojovic, D.; Kesic, L.; Obradovic, R. The effects of low level laser irradiation on gingival inflammation. Photomed. Laser Surg. 2010, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rybitwa, D.; Rahnama, M.; Wilczyński, S. Microorganisms colonising historical cardboard objects from the Auschwitz-Birkenau State Museum in Oświęcim, Poland and their disinfection with vaporised hydrogen peroxide (VHP). Int. Biodeterior. Biodegrad. 2020, 152, 104997. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Rahnama, M. Application of a Medical Diode Laser (810 nm) for Disinfecting Small Microbiologically Contaminated Spots on Degraded Collagenous Materials for Improved Biosafety in Objects of Exceptional Historical Value from the Auschwitz-Birkenau State Museum and Protection of Human Health. Front. Microbiol. 2020, 11, 596852. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Wilczyński, S.; Łobacz, M. Irradiation with medical diode laser as a new method of spot-elimination of microorganisms to preserve historical cellulosic objects and human health. Int. Biodeterior. Biodegrad. 2020, 154, 105055. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Rybitwa, D.; Wieczorek, K.; Michalczewski, G.; Podsiadły, E.; Łobacz, M. Decontamination of microbiologically contaminated abiotic porous surfaces in an oral surgery clinic using vaporised hydrogen peroxide (VHP). J. Environ. Health Sci. Eng. 2020, 18, 639–653. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic Profiles of Patients with Dental Prosthetic Treatment and Periodontitis before and after Photoactivation Therapy—Randomized Clinical Trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- Dai, S.; Xiao, G.; Dong, N.; Liu, F.; He, S.; Guo, Q. Bactericidal effect of a diode laser on Enterococcus faecalis in human primary teeth-an in vitro study. BMC Oral Health 2018, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, N.; Van Gogswaardt, D.; Conrads, G.; Apel, C.; Schubert, C.; Lampert, F. Diode Laser Radiation and Its Bactericidal Effect in Root Canal Wall Dentin. J. Clin. Laser Med. Surg. 2000, 18, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Meire, M.A.; De Prijck, K.; Coenye, T.; Nelis, H.J.; De Moor, R.J.G. Effectiveness of different laser systems to killEnterococcus faecalisin aqueous suspension and in an infected tooth model. Int. Endod. J. 2009, 42, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Nelson, A.E.; Heo, G.; Major, P.W. Surface chemical composition of human maxillary first premolar as assessed by X-ray photoelectron spectroscopy (XPS). Appl. Surf. Sci. 2008, 254, 6706–6709. [Google Scholar] [CrossRef]
- Zamudio-Ortega, C.M.; Contreras-Bulnes, R.; ScougallVilchis, R.J.; Morales-Luckie, R.A.; Olea-Mejía, O.F.; Rodríguez Vilchis, L.E. Morphological, chemical and structural characterisation of deciduous enamel: SEM, EDS, XRD, FTIR and XPS analysis. Eur. J. Paediatr. Dent. 2014, 15, 275–280. [Google Scholar]
- Omae, M.; Shinnou, Y.; Tanaka, K.; Abo, T.; Nakata, T.; Suzuki, K.; Hatsuoka, Y.; Iwata, N.; Yoshikawa, K.; Nishitani, Y.; et al. XPS analysis of the dentin irradiated by Er:YAG laser. Dent. Mater. J. 2009, 28, 471–476. [Google Scholar] [CrossRef][Green Version]
| No. | Microorganism | The Average Number on the Teeth [CFU/0.4 cm2] | Standard Deviation |
|---|---|---|---|
| 1 | Candida albicans | 3.12 × 105 | 2.55 × 104 |
| 2 | Candida guilliermondii | 5.59 × 103 | 1.41 × 101 |
| 3 | Escherichia coli | 1.65 × 107 | 2.12 × 106 |
| 4 | Haemophilus parainfluenzae | 1.44 × 108 | 9.05 × 106 |
| 5 | Klebsiella oxytoca | 3.88 × 107 | 2.26 × 106 |
| 6 | Neisseria subflava | 1.11 × 106 | 7.92 × 104 |
| 7 | Rothia dentocariosa | 1.65 × 106 | 9.05 × 104 |
| 8 | Rothia mucilaginosa | 7.90 × 104 | 4.24 × 103 |
| 9 | Streptococcus pneumoniae | 1.77 × 105 | 2.12 × 104 |
| Microorganisms | L1, t = 1 min | L2, t = 1 min | L3a, t = 1 × 15 s | L3b, t = 2 × 15 s | L3c, t = 3 × 15 s |
|---|---|---|---|---|---|
| Reduction [%] | |||||
| Candida albicans | 57.97 * | 67.82 * | 80.03 | 85.01 * | 93.80 * |
| Candida guilliermondii | 60.40 * | 62.88 * | 78.64 * | 83.79 * | 92.51 * |
| Haemophilus parainfluenzae | 69.04 * | 70.03 * | 86.30 * | 86.67 * | 88.02 * |
| Klebsiella oxytoca | 30.67 * | 48.20 * | 61.52 * | 68.49 * | 100.00 * |
| Rothia dentocariosa | 68.97 * | 72.43 * | 88.35 * | 92.90 * | 98.77 * |
| Rothia mucilaginosa | 78.09 * | 80.76 * | 91.19 * | 96.37 * | 100.00 * |
| Streptococcus pneumoniae | 66.67 * | 78.12 * | 98.73 * | 99.70 * | 99.91 * |
| Escherichia coli ATCC 13796 | 69.35 * | 74.56 * | 78.67 * | 94.70 * | 99.30 * |
| Staphylococcus aureus ATCC 23235 | 65.08 * | 70.07 * | 85.18 * | 97.17 * | 99.99 * |
| Spectral Band | Band C 1s | ||||
|---|---|---|---|---|---|
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| C 1s A | 284.11 | 10.2 | 284.10 | 11.9 | C=C sp2 |
| C 1s B | 284.71 | 45.1 | 284.70 | 55.9 | C–H |
| C 1s C | 285.30 | 19.9 | 285.29 | 21.6 | C–C sp3 |
| C 1s D | 286.11 | 8.5 | 286.10 | 2.9 | C–OH |
| C 1s E | 286.79 | 4.8 | 286.78 | 2.1 | C–O–C |
| C 1s F | 287.68 | 4.5 | 287.68 | 2.1 | C=O |
| C 1s G | 288.53 | 4.2 | 288.53 | 2.9 | –O–C=O |
| C 1s H | 289.30 | 3.0 | 289.30 | 0.5 | CO32− |
| Spectral band | Band O 1s | ||||
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| O 1s A | 530.95 | 16.17 | 530.97 | 18.2 | O–C=O |
| O 1s B | 531.77 | 58.18 | 531.62 | 54.3 | O=C |
| O 1s C | 532.61 | 15.89 | 532.56 | 22.8 | HO–C (C–(O)–C) |
| O 1s D | 533.48 | 9.77 | 533.67 | 4.7 | HO–C C–O–C |
| Spectral band | Band N 1s | ||||
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| N 1s A | 399.30 | 19.9 | 399.26 | 8.1 | C–NH2 (amina) |
| N 1s B | 400.07 | 80.1 | 400.06 | 91.9 | –N–(C=O) (amid) |
| Spectral band | Band Si 2p | ||||
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| Si 2p A | 102.01 | 100.0 | 101.99 | 100.0 | Si-O |
| Spectral band | Band Ca 2p | ||||
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| Ca 2p 3/2 | 347.44 | 50.7 | 347.43 | 50.7 | Ca3PO4, CaCO3 |
| Ca 2p 1/2 | 350.93 | 49.3 | 350.97 | 49.3 | |
| Spectral band | Band P 2p | ||||
| K | L | Phase | |||
| EB/eV | % At | EB/eV | % At | ||
| P 2p 1/2 | 133.93 | 49.5 | 133.70 | 49.5 | PO43− |
| P 2p 3/2 | 133.07 | 50.5 | 132.84 | 50.5 | |
| Roughness Parameter Ra (µm) | ||||
|---|---|---|---|---|
| Sample | K (No Exposure) | Tooth Surface after Irradiation L3a, t = 1 × 15 s | Tooth Surface after Irradiation L3b, t = 2 × 15 s | Tooth Surface after Irradiation L3c, t = 3 × 15 s |
| Measurement | 946 µm × 1261 µm | |||
| Average value | 12.294 | 10.677 | 11.332 | 4.283 |
| Measurement | 117 µm × 156 µm | |||
| Average value | 1.207 | 0.551 | 0.481 | 0.396 |
| Measurement | 47 µm × 62 µm | |||
| Average value | 0.452 | 0.248 | 0.256 | 0.257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyk-Bochenek, I.; Łobacz, M.; Wilczyński, S.; Rahnama, M.; Szulc, J.; Konka, A.; Wawrzyk, A. Evaluation of the Tooth Surface after Irradiation with Diode Laser Applied for Removal of Dental Microorganisms from Teeth of Patients with Gingivitis, Using X-ray Photoelectron (XPS) and Optical Profilometry (OP). J. Clin. Med. 2022, 11, 6840. https://doi.org/10.3390/jcm11226840
Wawrzyk-Bochenek I, Łobacz M, Wilczyński S, Rahnama M, Szulc J, Konka A, Wawrzyk A. Evaluation of the Tooth Surface after Irradiation with Diode Laser Applied for Removal of Dental Microorganisms from Teeth of Patients with Gingivitis, Using X-ray Photoelectron (XPS) and Optical Profilometry (OP). Journal of Clinical Medicine. 2022; 11(22):6840. https://doi.org/10.3390/jcm11226840
Chicago/Turabian StyleWawrzyk-Bochenek, Iga, Michał Łobacz, Sławomir Wilczyński, Mansur Rahnama, Justyna Szulc, Adam Konka, and Anna Wawrzyk. 2022. "Evaluation of the Tooth Surface after Irradiation with Diode Laser Applied for Removal of Dental Microorganisms from Teeth of Patients with Gingivitis, Using X-ray Photoelectron (XPS) and Optical Profilometry (OP)" Journal of Clinical Medicine 11, no. 22: 6840. https://doi.org/10.3390/jcm11226840
APA StyleWawrzyk-Bochenek, I., Łobacz, M., Wilczyński, S., Rahnama, M., Szulc, J., Konka, A., & Wawrzyk, A. (2022). Evaluation of the Tooth Surface after Irradiation with Diode Laser Applied for Removal of Dental Microorganisms from Teeth of Patients with Gingivitis, Using X-ray Photoelectron (XPS) and Optical Profilometry (OP). Journal of Clinical Medicine, 11(22), 6840. https://doi.org/10.3390/jcm11226840







