Influence of Metal Implants on Quantitative Evaluation of Bone Single-Photon Emission Computed Tomography/Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
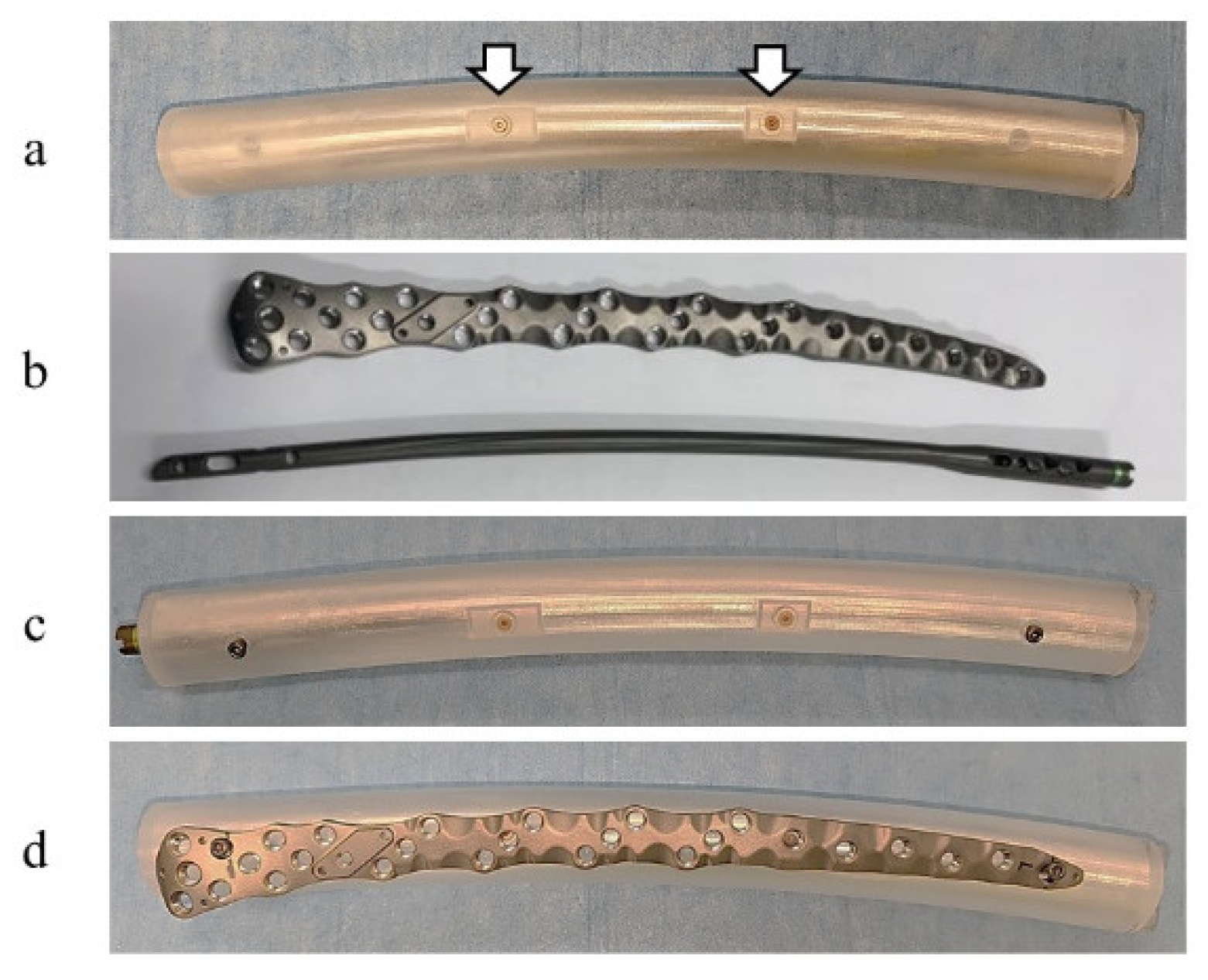
2.1. Phantom and Implants
2.2. SPECT/CT
2.3. SUV Measurements
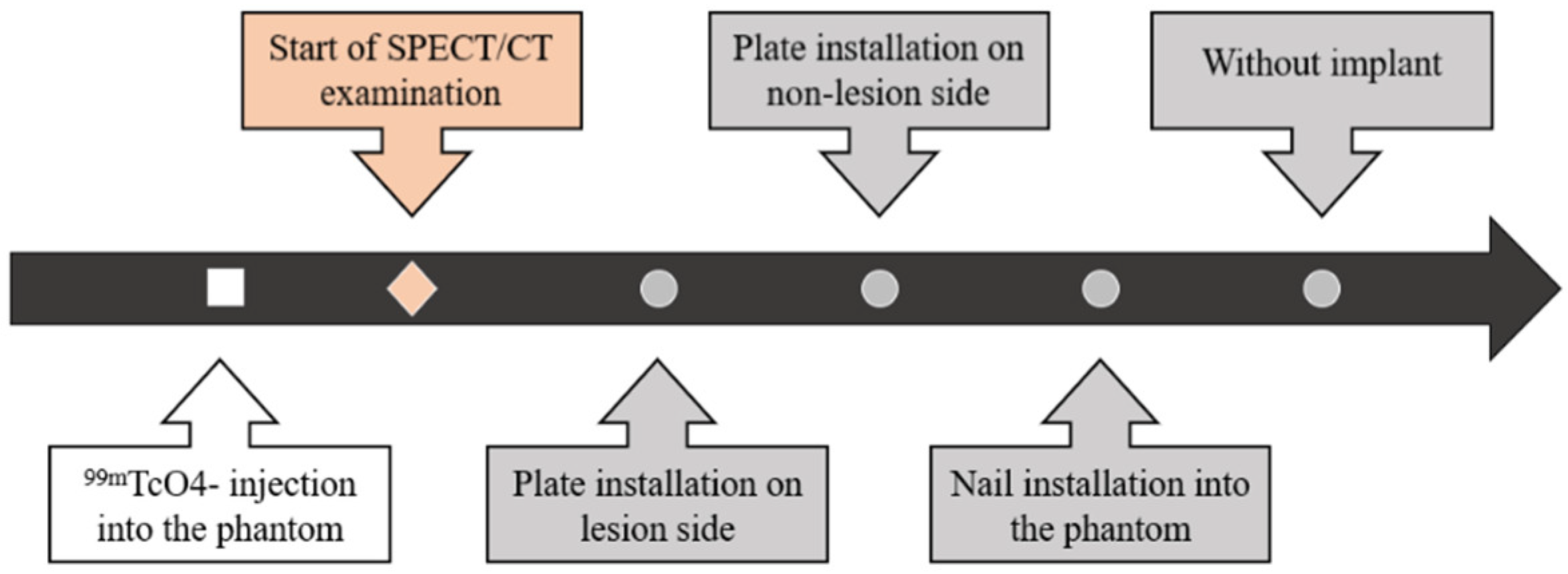
2.4. Experimental Procedure
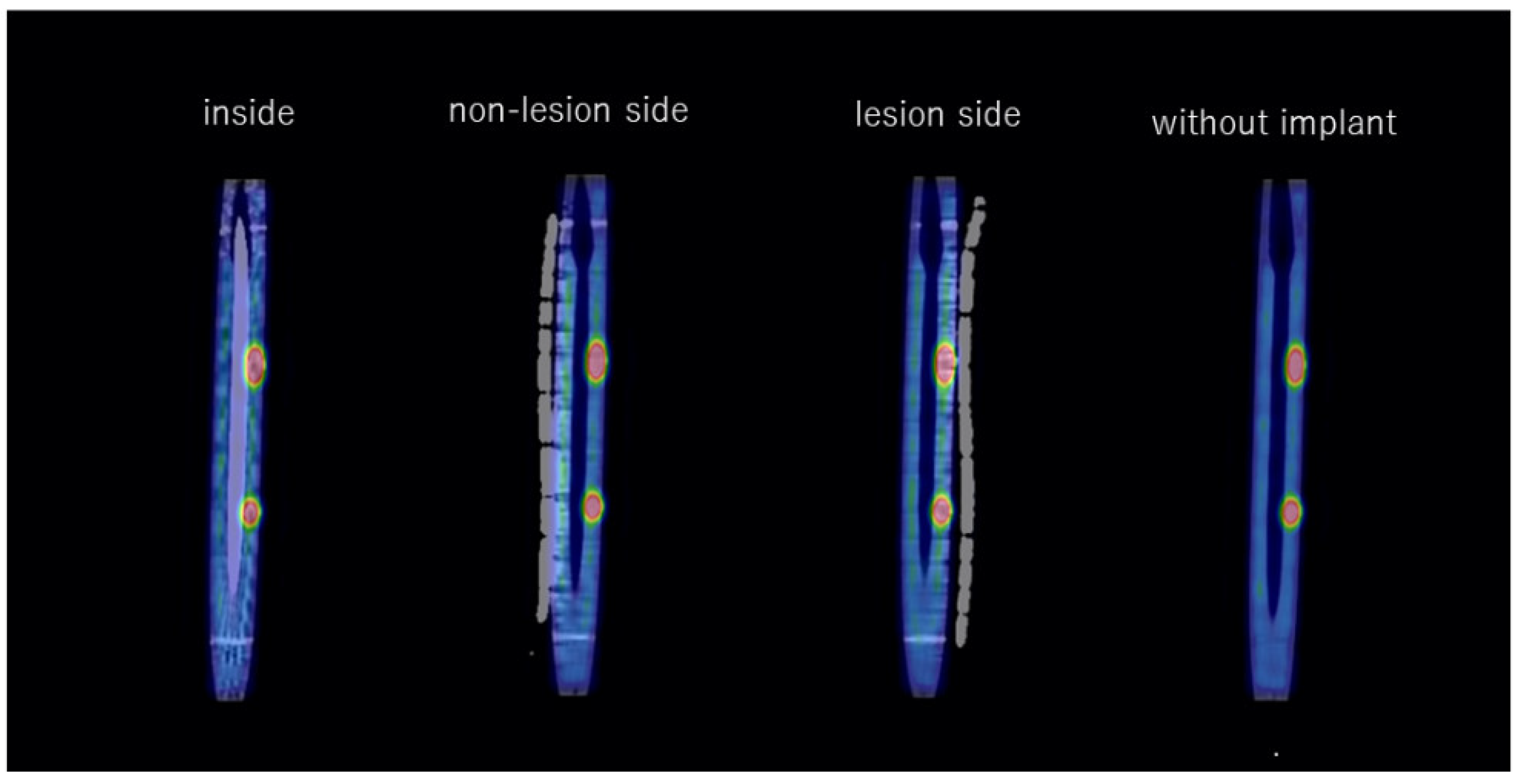
2.5. Evaluation
2.6. Statistical Analysis
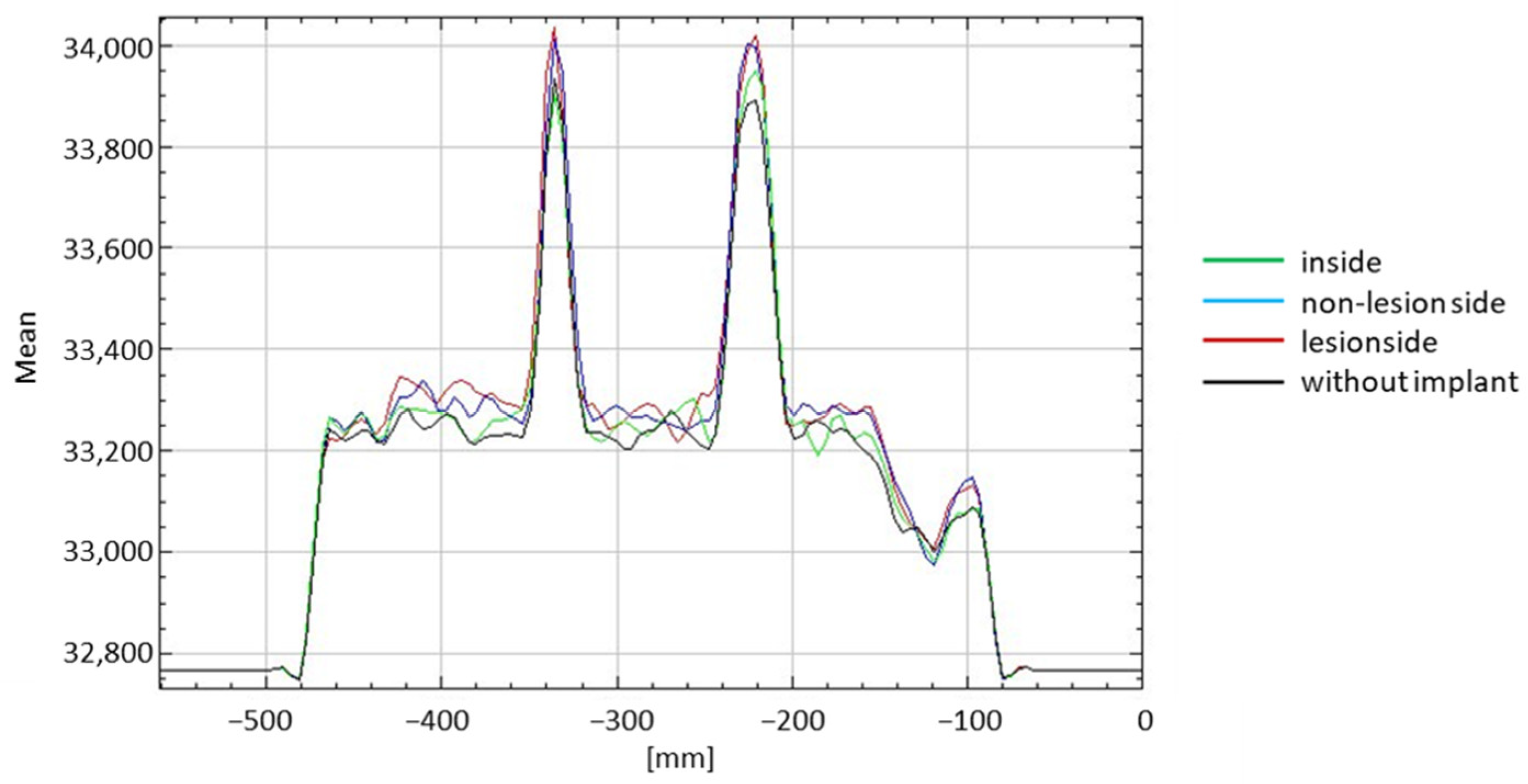
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zura, R.; Watson, J.T.; Einhorn, T.; Mehta, S.; Della Rocca, G.J.; Xiong, Z.; Wang, Z.; Jones, J.; Steen, R.G. An inception cohort analysis to predict nonunion in tibia and 17 other fracture locations. Injury 2017, 48, 1194–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, S.K. Fracture non-union: A review of clinical challenges and future research needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.I.; Keating, J.F.; Court-Brown, C.M. Cigarette smoking and open tibial fractures. Injury 2001, 32, 61–65. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Do, T.P.; Critchlow, C.W.; Dent, R.E.; Jick, S.S. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012, 83, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Takenaka, N.; Kobayashi, M.; Matsushita, T. Infra-isthmal fracture is a risk factor for nonunion after femoral nailing: A case-control study. J. Orthop. Sci. 2013, 18, 76–80. [Google Scholar] [CrossRef]
- Oe, K.; Zeng, F.; Fukui, T.; Nogami, M.; Murakami, T.; Matsumoto, T.; Kuroda, R.; Niikura, T. Quantitative bone single-photon emission computed tomography imaging for uninfected nonunion: Comparison of hypertrophic nonunion and non-hypertrophic nonunion. J. Orthop. Surg. Res. 2021, 16, 125. [Google Scholar] [CrossRef]
- Niikura, T.; Lee, S.Y.; Sakai, Y.; Nishida, K.; Kuroda, R.; Kurosaka, M. Comparison of radiographic appearance and bone scintigraphy in fracture nonunions. Orthopedics 2014, 37, e44–e50. [Google Scholar] [CrossRef] [Green Version]
- Duncan, I.; Ingold, N. The clinical value of xSPECT/CT bone versus SPECT/CT. A prospective comparison of 200 scans. Eur. J. Hybrid Imaging 2018, 2, 4. [Google Scholar] [CrossRef]
- Sumer, J.; Schmidt, D.; Ritt, P.; Lell, M.; Forst, R.; Kuwert, T.; Richter, R. SPECT/CT in patients with lower back pain after lumbar fusion surgery. Nucl. Med. Commun. 2013, 34, 964–970. [Google Scholar] [CrossRef]
- Mushtaq, N.; To, K.; Gooding, C.; Khan, W. Radiological imaging evaluation of the failing total hip replacement. Front. Surg. 2019, 6, 35. [Google Scholar] [CrossRef]
- Bhure, U.; Agten, C.; Lehnick, D.; Perez-Lago, M.D.S.; Beeres, F.; Link, B.C.; Strobel, K. Value of SPECT/CT in the assessment of necrotic bone fragments in patients with delayed bone healing or non-union after traumatic fractures. Br. J. Radiol. 2020, 93, 20200300. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; van der Bruggen, W.; Hug, U.; Gnanasegaran, G.; Kampen, W.U.; Kuwert, T.; Paycha, F.; van den Wyngaert, T. SPECT/CT in postoperative hand and wrist pain. Semin. Nucl. Med. 2018, 48, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Miwa, K.; Matsutomo, N.; Watanabe, Y.; Kato, T.; Shimada, H. Development of a novel body phantom with bone equivalent density for evaluation of Bone SPECT. Nihon Hoshasen Gijutsu Gakkai Zasshi 2015, 71, 1235–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarasekera, H.W.; Costa, M.L.; Parsons, N.; Achten, J.; Griffin, D.R.; Manktelow, S.; Williams, N.R. SPECT/CT bone imaging after hip resurfacing arthroplasty: Is it feasible to use CT attenuation correction in the presence of metal implants? Nucl. Med. Commun. 2011, 32, 289–297. [Google Scholar] [CrossRef]
- Chew, C.G.; Lewis, P.; Middleton, F.; van den Wijngaard, R.; Deshaies, A. Radionuclide arthrogram with SPECT/CT for the evaluation of mechanical loosening of hip and knee prostheses. Ann. Nucl. Med. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Miwa, K.; Matsutomo, N.; Ichikawa, H.; Kikuchi, A.; Shimada, H.; Narita, A.; Mori, K.; Fujino, K. Guideline on standardization of bone SPECT imaging. Jpn. J. Nucl. Med. Tech. 2017, 37, 517–530. [Google Scholar]
- Suzuki, A.; Koshida, K.; Matsubara, K. Adjustment of overestimated CT-based attenuation correction on bone SPECT/CT after hip-resurfacing arthroplasty. J. Nucl. Med. Technol. 2013, 41, 203–207. [Google Scholar] [CrossRef]
- Lai, P.J.; Hsu, Y.H.; Chou, Y.C.; Yeh, W.L.; Ueng, S.W.N.; Yu, Y.H. Augmentative antirotational plating provided a significantly higher union rate than exchanging reamed nailing in treatment for femoral shaft aseptic atrophic nonunion—Retrospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 127. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Noaman, H.H.; Soroor, Y.O.; Elsayed, M. Plate augmentation and bone grafting in treatment of femoral shaft nonunion initially fixed by intramedullary nail. SICOT J. 2022, 8, 19. [Google Scholar] [CrossRef]
- Mittal, K.K.; Gupta, H.; Kaushik, N. Reunion of post nail aseptic non-union of diaphyseal femoral fractures by augmentation plating, decortication and bone grafting—Replacement for exchange nailing. Injury 2021, 52, 1529–1533. [Google Scholar] [CrossRef]
- Kubik, J.F.; Bornes, T.D.; Gausden, E.B.; Klinger, C.E.; Wellman, D.S.; Helfet, D.L. Surgical outcomes of dual-plate fixation for periprosthetic femur fractures around a stable hip arthroplasty stem. Arch. Orthop. Trauma Surg. 2021, 142, 3605–3611. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.Y.; Cong, Y.; Shi, D.; Lu, Y.H.; Niu, Y.F.; Xu, H.D. Augmentative locking plate with autologous bone grafting for distal femoral nonunion subsequent to failed retrograde intramedullary nailing. Acta Orthop. Traumatol. Turc. 2016, 50, 393–399. [Google Scholar] [CrossRef] [PubMed]

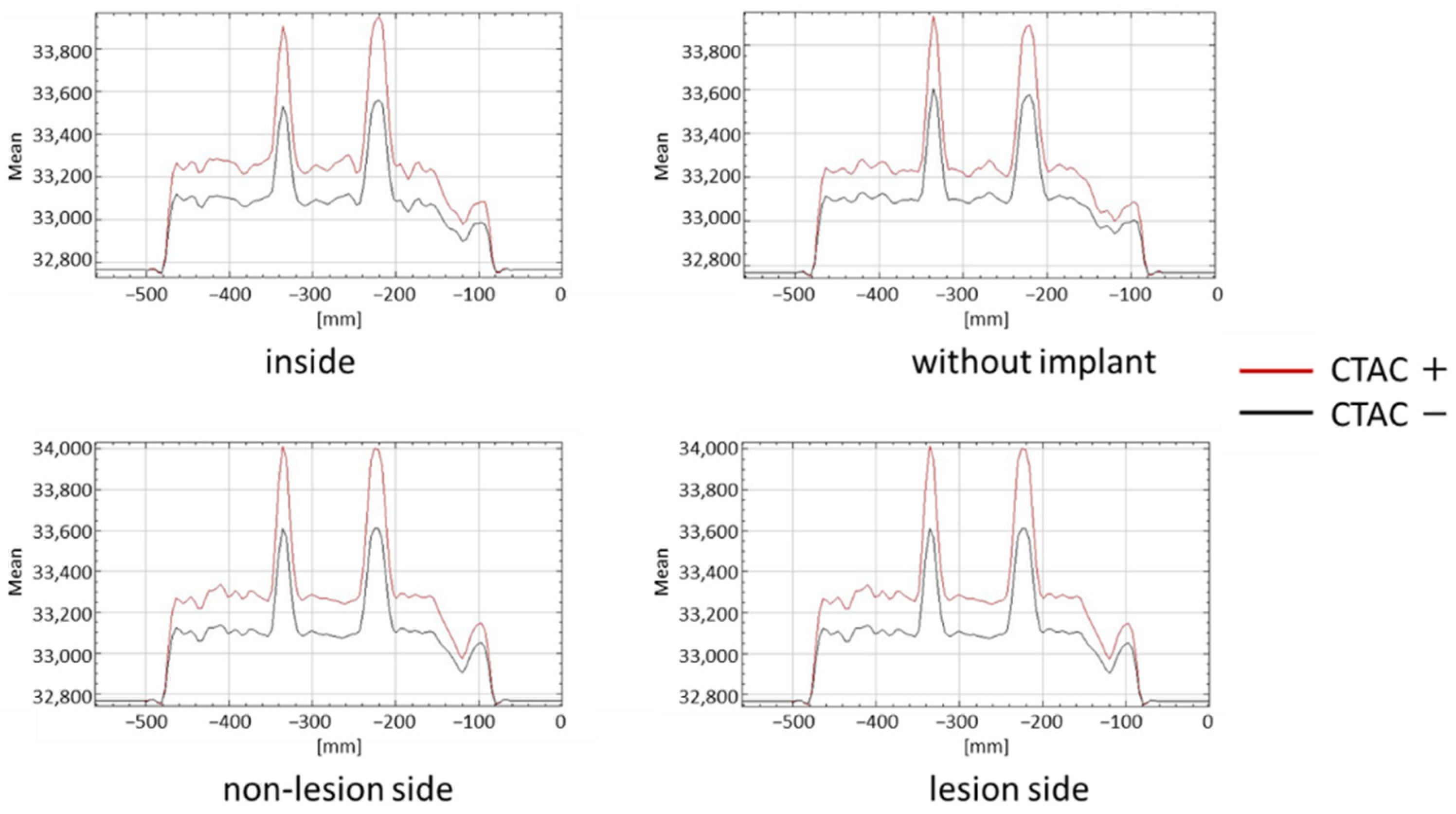

| Inside | Lesion Side | Non-Lesion Side | |
|---|---|---|---|
| Without implant | p < 0.001 | p < 0.001 | p < 0.001 |
| Lesion side | p = 0.087 | ||
| Non-lesion side | p < 0.001 | p = 0.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oe, K.; Zeng, F.; Niikura, T.; Fukui, T.; Sawauchi, K.; Matsumoto, T.; Nogami, M.; Murakami, T.; Kuroda, R. Influence of Metal Implants on Quantitative Evaluation of Bone Single-Photon Emission Computed Tomography/Computed Tomography. J. Clin. Med. 2022, 11, 6732. https://doi.org/10.3390/jcm11226732
Oe K, Zeng F, Niikura T, Fukui T, Sawauchi K, Matsumoto T, Nogami M, Murakami T, Kuroda R. Influence of Metal Implants on Quantitative Evaluation of Bone Single-Photon Emission Computed Tomography/Computed Tomography. Journal of Clinical Medicine. 2022; 11(22):6732. https://doi.org/10.3390/jcm11226732
Chicago/Turabian StyleOe, Keisuke, Feibi Zeng, Takahiro Niikura, Tomoaki Fukui, Kenichi Sawauchi, Tomoyuki Matsumoto, Munenobu Nogami, Takamichi Murakami, and Ryosuke Kuroda. 2022. "Influence of Metal Implants on Quantitative Evaluation of Bone Single-Photon Emission Computed Tomography/Computed Tomography" Journal of Clinical Medicine 11, no. 22: 6732. https://doi.org/10.3390/jcm11226732
APA StyleOe, K., Zeng, F., Niikura, T., Fukui, T., Sawauchi, K., Matsumoto, T., Nogami, M., Murakami, T., & Kuroda, R. (2022). Influence of Metal Implants on Quantitative Evaluation of Bone Single-Photon Emission Computed Tomography/Computed Tomography. Journal of Clinical Medicine, 11(22), 6732. https://doi.org/10.3390/jcm11226732







