Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Verdoia, M.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Neutrophil to Lymphocyte Ratio and the Ex-tent of Coronary Artery Disease: Results From a Large Cohort Study. Angiology 2016, 67, 75–82. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Verdoia, M.; Cassetti, E.; Schaffer, A.; Cavallino, C.; Bolzani, V.; Marino, P.; Novara Atherosclerosis Study Group (NAS). High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian popu-lation. J. Thromb. Thrombolysis 2011, 31, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Sartori, C.; Barbieri, L.; Cassetti, E.; Marino, P.; Galasso, G.; De Luca, G. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur. J. Clin. Investig. 2014, 44, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Verdoia, M.; Schaffer, A.; Marino, P.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: A single-centre study. Atherosclerosis 2015, 241, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ockene, I.S.; Miller, N.H. Cigarette smoking, cardiovascular disease, and stroke: A statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997, 96, 3243–3247. [Google Scholar] [CrossRef]
- Barbash, G.I.; White, H.D.; Modan, M.; Diaz, R.; Hampton, J.R.; Heikkila, J.; Kristinsson, A.; Moulopoulos, S.; Paolasso, E.A.; Van der Werf, T. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation 1993, 87, 53–58. [Google Scholar] [CrossRef]
- Helmers, C. Short and long-term prognostic indices in acute myocardial infarction. A study of 606 patients initially treated in a coronary care unit. Acta Med. Scand. Suppl. 1973, 555, 7–26. [Google Scholar]
- Kelly, T.L.; Gilpin, E.; Ahnve, S.; Henning, H.; Ross, J. Smoking status at the time of acute myocardial infarction and subsequent prognosis. Am. Heart J. 1985, 110, 535–541. [Google Scholar]
- Sparrow, D.; Dawber, T.R. The influence of cigarette smoking on prognosis after a first myocardial infarction. A report from the Framingham Study. J. Chronic Dis. 1978, 31, 425–432. [Google Scholar] [CrossRef]
- Jaatun, H.J.; Sutradhar, S.C.; Dickstein, K. Comparison of mortality rates after acute my-ocardial infarction in smokers vs. nonsmokers. Am. J. Cardiol. 2004, 94, 632–636, A639. [Google Scholar] [CrossRef]
- Ali, S.F.; Smith, E.E.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J. Am. Heart Assoc. 2013, 2, e000171. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Nunez, E.; Yancy, C.W.; Young, J.B. A smoker’s paradox in patients hospitalized for heart failure: Findings from OPTIMIZE-HF. Eur. Heart J. 2008, 29, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Jain, D.; Sule, S.; Ahmed, A.; Iwai, S.; et al. Relation of smoking status to outcomes after cardiopulmonary resuscitation for in-hospital cardiac arrest. Am. J. Cardiol. 2014, 114, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; Smith, E.E.; Reeves, M.J.; Zhao, X.; Xian, Y.; Hernandez, A.F.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H. Smoking paradox in patients hospitalized with coronary artery disease or acute ischemic stroke: Findings from Get with The Guidelines. Circ. Cardiovasc. Qual. Outcomes 2015, 8, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Zahger, D.; Cercek, B.; Cannon, C.P.; Jordan, M.; Davis, V.; Braunwald, E.; Shah, P.K. How do smokers differ from nonsmokers in their response to thrombolysis? (the TIMI-4 trial). Am. J. Cardiol. 1995, 75, 232–236. [Google Scholar] [CrossRef]
- Lundergan, C.F.; Reiner, J.S.; McCarthy, W.F.; Coyne, K.S.; Califf, R.M.; Ross, A.M. Clinical predictors of early infarct-related artery patency following thrombolytic therapy: Importance of body weight, smoking history, infarct-related artery and choice of thrombolytic regimen: The GUSTO-I experience. Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 1998, 32, 641–647. [Google Scholar]
- de Chillou, C.; Riff, P.; Sadoul, N.; Ethevenot, G.; Feldmann, L.; Isaaz, K.; Simon, J.P.; Boursier, M.; Khalife, K.; Thisse, J.Y.; et al. Influence of cigarette smoking on rate of reopening of the infarct-related coronary artery after myocardial infarction: A multivariate analysis. J. Am. Coll. Cardiol. 1996, 27, 1662–1668. [Google Scholar] [CrossRef]
- Ferreiro, J.L.; Bhatt, D.L.; Ueno, M.; Bauer, D.; Angiolillo, D.J. Impact of smoking on long-term outcomes in patients with atherosclerotic vascular disease treated with aspirin or clopidogrel: Insights from the CAPRIE trial (Clopidogrel Vs. Aspirin in Patients at Risk of Ischemic Events). J. Am. Coll. Cardiol. 2014, 63, 769–777. [Google Scholar] [CrossRef]
- Berger, J.S.; Bhatt, D.L.; Steinhubl, S.R.; Shao, M.; Steg, P.G.; Montalescot, G.; Hacke, W.; Fox, K.A.; Lincoff, A.M.; Topol, E.J.; et al. Smoking, clopidogrel, and mortality in patients with established cardiovascular disease. Circulation 2009, 120, 2337–2344. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Logan, D.K.; Kereiakes, D.J.; Lasseter, K.C.; White, A.; Angiolillo, D.J.; Nolin, T.D.; Maa, J.F.; Bailey, W.L.; et al. The influence of smoking status on the pharmacokinetics and pharmaco-dynamics of clopidogrel and prasugrel: The PARADOX study. J. Am. Coll. Cardiol. 2013, 62, 505–512. [Google Scholar] [CrossRef]
- Cornel, J.H.; Ohman, E.M.; Neely, B.; Clemmensen, P.; Sritara, P.; Zamoryakhin, D.; Armstrong, P.W.; Prabhakaran, D.; White, H.D.; Fox, K.A.; et al. Impact of smoking status on platelet function and clinical outcomes with prasugrel vs. clopidogrel in patients with acute coronary syndromes managed without revascularization: Insights from the TRILOGY ACS trial. Am. Heart J. 2014, 168, 76–87.e71. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Albaghdadi, M.S.; Meraj, P.M.; Schmidt, C.; Garberich, R.; Jaffer, F.A.; Dixon, S.; Rade, J.J.; Tannenbaum, M.; Chambers, J.; et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2871–2872. [Google Scholar] [CrossRef]
- Tam, C.F.; Cheung, K.S.; Lam, S.; Wong, A.; Yung, A.; Sze, M.; Lam, Y.M.; Chan, C.; Tsang, T.C.; Tsui, M.; et al. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment-Elevation Myocardial Infarction Care in Hong Kong, China. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006631. [Google Scholar] [CrossRef]
- Xiang, D.; Xiang, X.; Zhang, W.; Yi, S.; Zhang, J.; Gu, X.; Xu, Y.; Huang, K.; Su, X.; Yu, B.; et al. Management and Outcomes of Patients with STEMI During the COVID-19 Pandemic in China. J. Am. Coll. Cardiol. 2020, 76, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Verdoia, M.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Ferrer, G.R.; Ganyukov, V.; Wojakowski, W.; et al. Impact of COVID-19 Pandemic on Mechanical Reperfusion for Patients With STEMI. J. Am. Coll. Cardiol. 2020, 76, 2321–2330. [Google Scholar] [CrossRef]
- De Luca, G.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Roura IFerrer, G.; Ganyukov, V.; Wojakowski, W.; von Birgelen, C.; et al. Impact of COVID-19 pandemic and diabetes on mechanical reperfusion in patients with STEMI: Insights from the ISACS STEMI COVID 19 Registry. Cardiovasc. Diabetol. 2020, 19, 215. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: Every minute of delay counts. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; van’t Hof, A.W.; de Boer, M.J.; Ottervanger, J.P.; Hoorntje, J.C.; Gosselink, A.T.; Dambrink, J.H.; Zijlstra, F.; Suryapranata, H. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur. Heart J. 2004, 25, 1009–1013. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- De Luca, G.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Zimbakov, Z.; Cercek, M.; Jensen, L.O.; Loh, P.H.; Calmac, L.; et al. COVID-19 pandemic, mechanical reperfusion and 30-day mortality in ST elevation myocardial infarction. Heart 2021, 108, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 1997, 127, 757–763. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; van’t Hof, A.W.; Hoorntje, J.C.; Gosselink, A.M.; Dambrink, J.H.E.; de Boer, M.J. Impact of statin therapy at discharge on 1-year mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty. Atherosclerosis 2006, 189, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristics (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef]
- Barbash, G.I.; White, H.D.; Modan, M.; Diaz, R.; Hampton, J.R.; Heikkila, J.; Kristinsson, A.; Moulopoulos, S.; Paolasso, E.A.; Van der Werf, T. Acute myocardial infarction in the young—The role of smoking. The Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Eur. Heart J. 1995, 16, 313–316. [Google Scholar]
- Grines, C.L.; Topol, E.J.; O’Neill, W.W.; George, B.S.; Kereiakes, D.; Phillips, H.R.; Leimberger, J.D.; Woodlief, L.H.; Califf, R.M. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation 1995, 91, 298–303. [Google Scholar] [CrossRef]
- Gottlieb, S.; Boyko, V.; Zahger, D.; Balkin, J.; Hod, H.; Pelled, B.; Stern, S.; Behar, S. Smoking and prognosis after acute myocardial infarction in the thrombolytic era (Israeli Thrombolytic National Survey). J. Am. Coll. Cardiol. 1996, 28, 1506–1513. [Google Scholar] [CrossRef][Green Version]
- McGill, H.C. The cardiovascular pathology of smoking. Am. Heart J. 1988, 115, 250–257. [Google Scholar] [CrossRef]
- Sambola, A.; Osende, J.; Hathcock, J.; Degen, M.; Nemerson, Y.; Fuster, V.; Crandall, J.; Badimon, J.J. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation 2003, 107, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, D.K.; Laurent, P.; Janoff, A. Cigarette smoke contains anticoagulants against fibrin aggregation and factor XIIIa in plasma. Science 1982, 217, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.J.; Martinezclark, P.; Rahman, A.M.; Ray, K.K.; Karmpaliotis, D.; Murphy, S.A.; Giugliano, R.P.; Cannon, C.P.; Antman, E.M.; Roe, M.T.; et al. Association of smoking with improved myocardial perfusion and the angiographic characterization of myocardial tissue perfusion after fibrinolytic therapy for ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 321–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Redfors, B.; Furer, A.; Selker, H.P.; Thiele, H.; Patel, M.R.; Chen, S.; Udelson, J.E.; Ohman, E.M.; Eitel, I.; Granger, C.B.; et al. Effect of Smoking on Outcomes of Primary PCI in Patients With STEMI. J. Am. Coll. Cardiol. 2020, 75, 1743–1754. [Google Scholar] [CrossRef]
- Steele, L.; Palmer, J.; Lloyd, A.; Fotheringham, J.; Iqbal, J.; Grech, E.D. The impact of smoking on mortality after acute ST-segment elevation myocardi-al infarction treated with primary percutaneous coronary intervention: A retrospective cohort outcome study at 3 years. J. Thromb. Thrombolysis 2019, 47, 520–526. [Google Scholar] [CrossRef]
- Gupta, T.; Kolte, D.; Khera, S.; Harikrishnan, P.; Mujib, M.; Aronow, W.S.; Jain, D.; Ahmed, A.; Cooper, H.A.; Frishman, W.H.; et al. Smoker’s Paradox in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2016, 5, e003370. [Google Scholar] [CrossRef]
- Goto, K.; Nikolsky, E.; Lansky, A.J.; Dangas, G.; Witzenbichler, B.; Parise, H.; Guagliumi, G.; Kornowski, R.; Claessen, B.E.; Fahy, M.; et al. Impact of smoking on outcomes of patients with ST-segment elevation myocardial infarction (from the HORIZONS-AMI Trial). Am. J Cardiol. 2011, 108, 1387–1394. [Google Scholar] [CrossRef]
- Desai, N.R.; Mega, J.L.; Jiang, S.; Cannon, C.P.; Sabatine, M.S. Interaction between cigarette smoking and clinical benefit of clopidogrel. J. Am. Coll. Cardiol. 2009, 53, 1273–1278. [Google Scholar] [CrossRef]
- Yousef, A.M.; Arafat, T.; Bulatova, N.R.; Al-Zumyli, R. Smoking behaviour modulates pharmacokinetics of orally administered clopidogrel. J. Clin. Pharm. Ther. 2008, 33, 439–449. [Google Scholar] [CrossRef]
- Shanker, G.; Kontos, J.L.; Eckman, D.M.; Wesley-Farrington, D.; Sane, D.C. Nicotine up-regulates the expression of P2Y12 on vascular cells and megakaryoblasts. J. Thromb. Thrombolysis 2006, 22, 213–220. [Google Scholar] [CrossRef]


| Variable | Active Smokers | Previous Smokers | Non-Smokers | p Value |
|---|---|---|---|---|
| (n = 6819) | (n = 2099) | (n = 7165) | ||
| Age (median, IQR) | 58 (51–65) | 67 (59–75) | 67 (58–77) | <0.001 |
| (54–72) | (54–72) | |||
| Age > 75 year—n (%) | 410 (6.0) | 533 (25.4) | 2104 (29.4) | <0.001 |
| Male gender—n (%) | 5538 (81.2) | 1773 (84.5) | 4853 (67.7) | <0.001 |
| Diabetes mellitus—n (%) | 1324 (19.4) | 502 (23.9) | 1986 (27.7) | <0.001 |
| Hypertension—n (%) | 3252 (47.7) | 1280 (61.0) | 4281 (59.7) | <0.001 |
| Hypercholesterolemia—n (%) | 2579 (37.8) | 1033 (49.2) | 2741 (38.3) | <0.001 |
| Family history of CAD—n (%) | 1667 (24.4) | 495 (23.6) | 1136 (15.9) | < 0.001 |
| Previous STEMI—n (%) | 571 (8.4) | 335 (16) | 637 (8.9) | <0.001 |
| Previous PCI—n (%) | 722 (10.6) | 432 (20.6) | 839 (11.7) | <0.001 |
| Previous CABG—n (%) | 57 (0.8) | 73 (3.5) | 142 (2.0) | <0.001 |
| Referral to primary PCI hospital | ||||
| Type | ||||
| Ambulance (from community)—n (%) | 3328 (48.8) | 1074 (51.2) | 3336 (46.6) | <0.001 |
| Direct access—n (%) | 1820 (26.7) | 512 (24.4) | 2181 (30.4) | |
| Spoke—n (%) | 1671 (24.5) | 513 (24.4) | 1648 (23.0) | |
| Time delays | ||||
| Ischemia time, median (25–75th) | 190 (10–350) | 208 (128–379) | 221 (130–400) | <0.001 |
| Total ischemia time | ||||
| <6 h—n (%) | 5228 (76.7) | 1550 (73.8) | 5144 (71.8) | <0.001 |
| 6–12 h—n (%) | 973 (14.3) | 328((15.6) | 1198 (16.7) | |
| 12–24 h—n (%) | 415 (6.1) | 142 (6.8) | 531 (7.4) | |
| >24 h—n (%) | 2.3 (3.0) | 79 (3.8) | 292 (4.1) | |
| Total ischemia time > 12 h—n (%) | 618 (9.1) | 221 (10.5) | 823 (11.5) | <0.001 |
| Door-to-balloon time, median (25–75th) | 40 (25–60) | 38 (21–65) | 40 (25–73) | <0.001 |
| Door-to-balloon time | ||||
| <30 min—n (%) | 2827 (41.5) | 887 (42.3) | 2719 (37.9) | <0.001 |
| 30–60 min—n (%) | 2305 (33.8) | 636 (30.3) | 2318 (32.4) | |
| >60 min—n (%) | 1687 (24.7) | 576 (27.4) | 2128 (29.7) | |
| Door-to-balloon time > 30 min (%)—n (%) | 3992 (58.5) | 1212 (57.7) | 4446 (62.1) | <0.001 |
| Clinical presentation | ||||
| Anterior STEMI—n (%) | 2994 (43.9) | 869 (41.4) | 3583 (50.0) | <0.001 |
| Out-of-hospital cardiac arrest—n (%) | 374 (5.5) | 91 (4.3) | 491 (6.9) | <0.001 |
| Cardiogenic shock—n (%) | 408 (6.0) | 157 (7.5) | 603 (8.4) | <0.001 |
| Rescue PCI for failed thrombolysis—n (%) | 579 (8.5) | 77 (3.7) | 443 (6.2) | <0.001 |
| In-hospital RASI therapy—n (%) | 3864 (56.7) | 1223 (58.3) | 3810 (53.2) | <0.001 |
| COVID positivity (%) | 28 (0.4%) | 16 (0.8%) | 65 (0.9%) | <0.001 |
| Active Smokers | Previous Smokers | Non-Smokers | p Value | |
|---|---|---|---|---|
| (n = 6819) | (n = 2099) | (n = 7165) | ||
| Radial Access (%) | 5289 (77.6) | 1698 (80.9) | 5281 (73.7) | <0.001 |
| Culprit vessel | ||||
| Left main—n (%) | 82 (1.2) | 49 (2.3) | 121 (1.7) | <0.001 |
| Left anterior descending artery—n (%) | 2976 (43.6) | 852 (40.6) | 3530 (49.3) | |
| Circumflex—n (%) | 1064 (15.6) | 362 (17.2) | 924 (12.9) | |
| Right coronary artery—n (%) | 2664 (39.1) | 812 (38.7) | 2525 (35.2) | |
| Anterolateral branch—n (%) | 12 (0.2) | 7 (0.3) | 22 (0.3) | |
| SVG n (%) | 20 (0.3) | 17 (0.8) | 42 (0.6) | |
| In-stent thrombosis—n (%) | 238 (3.5) | 127 (6.1) | 267 (3.7) | <0.001 |
| Multivessel disease—n (%) | 3145 (46.1) | 1062 (50.6) | 3679 (51.3) | <0.001 |
| Preprocedural TIMI 0 flow—n (%) | 4632 (67.9) | 1353 (64.5) | 4746 (66.2) | 0.007 |
| Thrombectomy—n (%) | 1112 (16.3) | 342 (16.3) | 1109 (15.5) | 0.36 |
| Stenting—n (%) | 6391 (93.7) | 1929 (91.9) | 6447 (90) | <0.001 |
| Drug-eluting stent—n (%) | 6150 (90.2) | 1858 (88.5) | 6246 (87.2) | <0.001 |
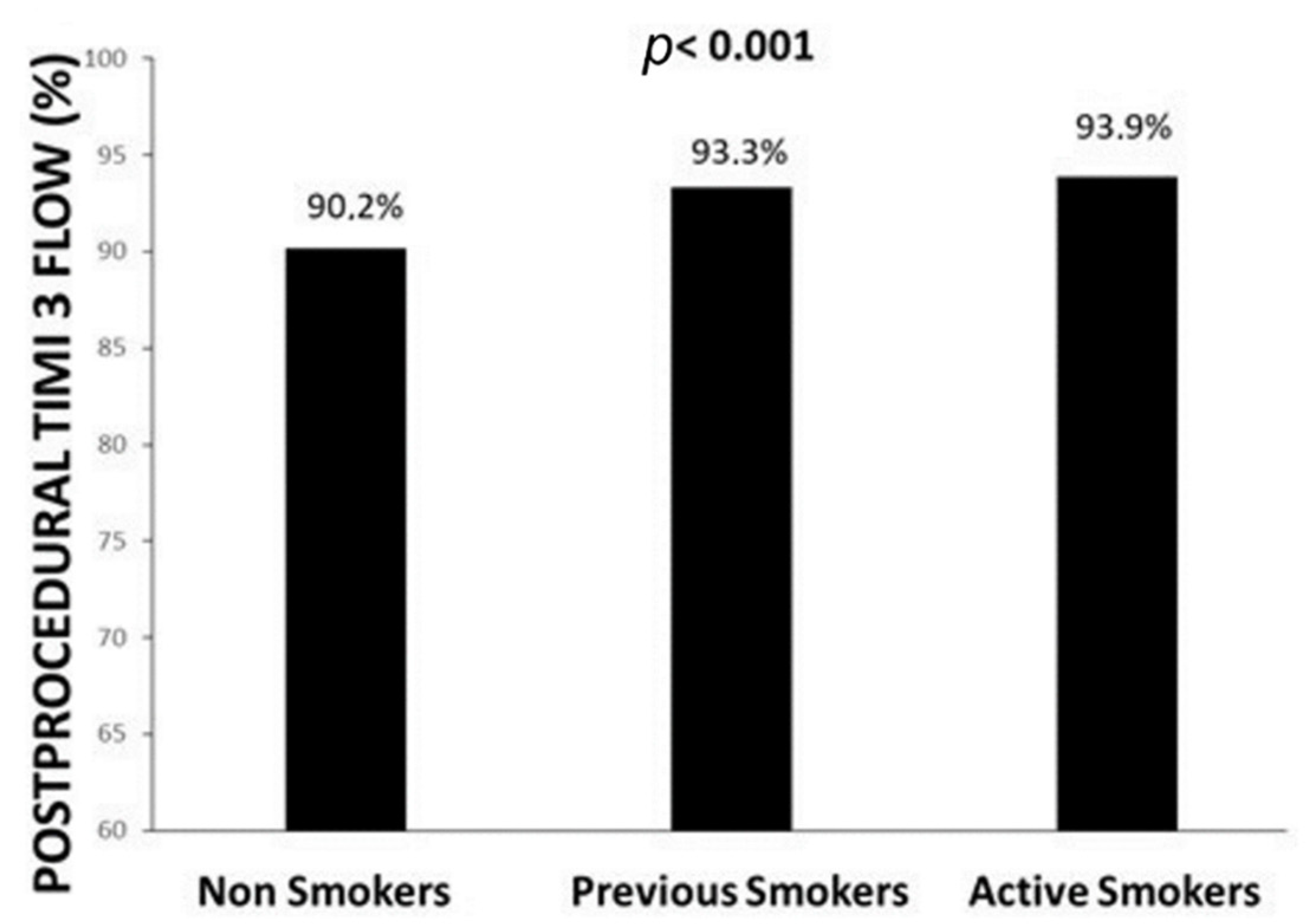
| Postprocedural TIMI 3 flow—n (%) | 6402 (93.9) | 1958 (93.3) | 6461 (90.2) | <0.001 |
| Gp IIb-IIIa inhibitors/cangrelor—n (%) | 1404 (20.6) | 441 (21.0) | 1422 (19.8) | 0.38 |
| Bivalirudin—n (%) | 18 (0.3) | 9 (0.4) | 25 (0.3) | 0.44 |
| Mechanical support—n (%) | 164 (2.4.) | 68 (3.2) | 265 (3.7) | <0.001 |
| Additional PCI | ||||
| During the index procedure—n (%) | 627 (9.2) | 260 (12.4) | 689 (9.6) | 0.001 |
| Staged—n (%) | 716 (10.5) | 223 (10.6) | 747 (10.4) | |
| DAPT therapy—n (%) | 6769 (99.3) | 2071 (98.7) | 7065 (98.6) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, G.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Zimbakov, Z.; Cercek, M.; Jensen, L.O.; Loh, P.H.; Calmac, L.; et al. Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry. J. Clin. Med. 2022, 11, 6722. https://doi.org/10.3390/jcm11226722
De Luca G, Algowhary M, Uguz B, Oliveira DC, Ganyukov V, Zimbakov Z, Cercek M, Jensen LO, Loh PH, Calmac L, et al. Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry. Journal of Clinical Medicine. 2022; 11(22):6722. https://doi.org/10.3390/jcm11226722
Chicago/Turabian StyleDe Luca, Giuseppe, Magdy Algowhary, Berat Uguz, Dinaldo C. Oliveira, Vladimir Ganyukov, Zan Zimbakov, Miha Cercek, Lisette Okkels Jensen, Poay Huan Loh, Lucian Calmac, and et al. 2022. "Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry" Journal of Clinical Medicine 11, no. 22: 6722. https://doi.org/10.3390/jcm11226722
APA StyleDe Luca, G., Algowhary, M., Uguz, B., Oliveira, D. C., Ganyukov, V., Zimbakov, Z., Cercek, M., Jensen, L. O., Loh, P. H., Calmac, L., Ferrer, G. R. i., Quadros, A., Milewski, M., Scotto D’Uccio, F., von Birgelen, C., Versaci, F., Ten Berg, J., Casella, G., Wong Sung Lung, A., ... Verdoia, M. (2022). Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry. Journal of Clinical Medicine, 11(22), 6722. https://doi.org/10.3390/jcm11226722












