Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patienst Recruitment
2.2. Investigations
2.2.1. General Investigations
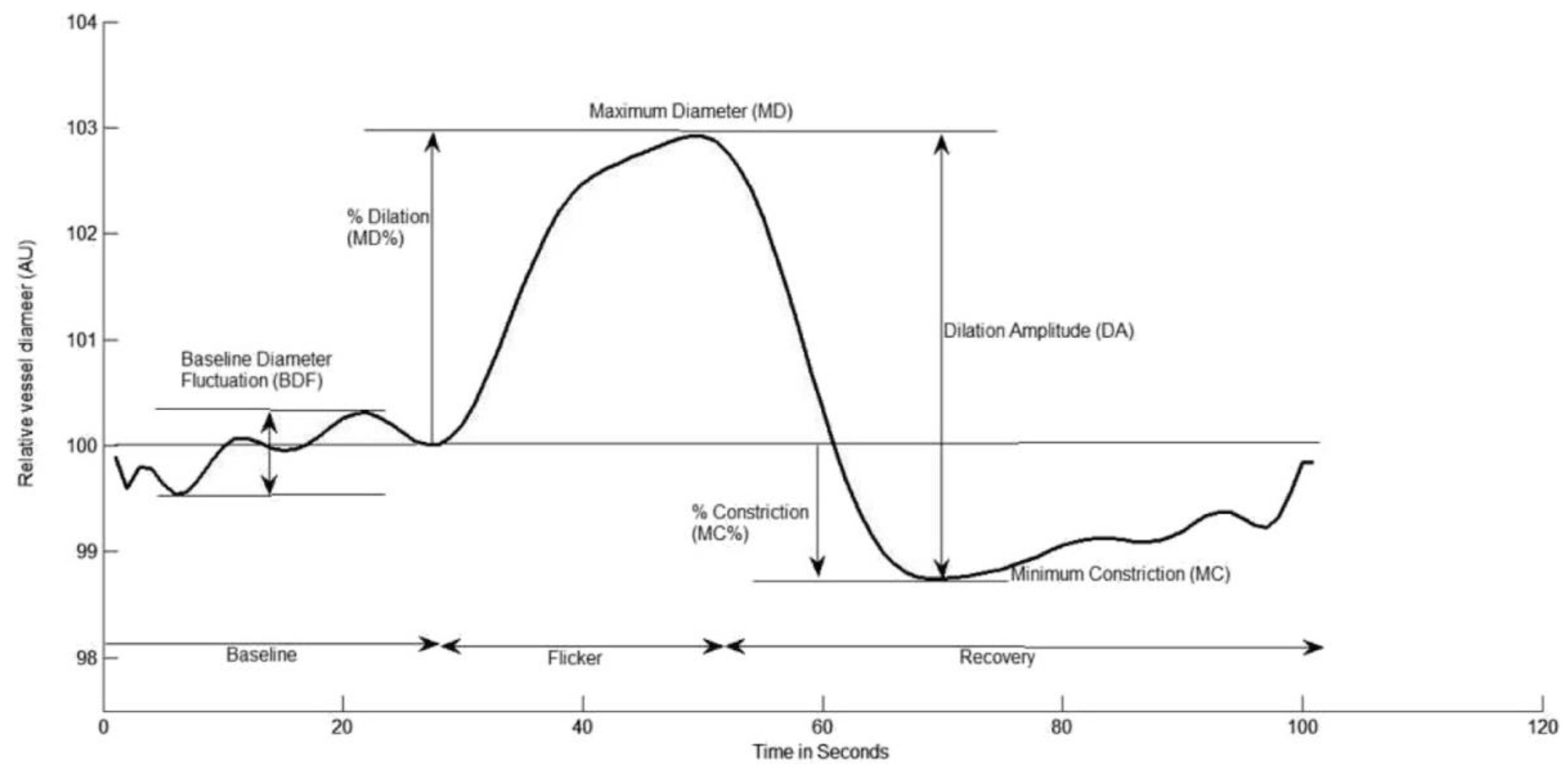
2.2.2. Dynamic Retinal Vessel Analysis (DVA)
2.3. Statistical Analysis
3. Results
3.1. Dynamic Retinal Vessel Analysis
3.1.1. Arterial Response
3.1.2. Venous Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, J.Y.; Kim, H.J.; Park, Y.H.; Park, T.K.; Park, E.C.; Kim, C.Y.; Lee, S.H. Association between Open-Angle Glaucoma and the Risks of Alzheimer’s and Parkinson’s Diseases in South Korea: A 10-Year Nationwide Cohort Study. Sci. Rep. 2018, 8, 11161. [Google Scholar] [CrossRef]
- Bayer, A.U.; Ferrari, F.; Erb, C. High Occurrence Rate of Glaucoma among Patients with Alzheimer’s Disease. Eur. Neurol. 2002, 47, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawakami, H.; Kanamoto, T.; Kato, T.; Yokoyama, T.; Sasaki, K.; Izumi, Y.; Matsumoto, M.; Mishima, H.K. High Frequency of Open-Angle Glaucoma in Japanese Patients with Alzheimer’s Disease. J. Neurol. Sci. 2006, 246, 79–83. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J. The Cell and Molecular Biology of Glaucoma: Common Neurodegenerative Pathways and Relevance to Glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Chitranshi, N.; den Haan, J.; Mirzaei, M.; You, Y.; Lim, J.K.; Basavarajappa, D.; Godinez, A.; Di Angelantonio, S.; Sachdev, P.; et al. Retinal Changes in Alzheimer’s Disease— Integrated Prospects of Imaging, Functional and Molecular Advances. Prog. Retin. Eye Res. 2021, 82, 100899. [Google Scholar] [CrossRef] [PubMed]
- Colligris, P.; Perez De Lara, M.J.; Colligris, B.; Pintor, J. Ocular Manifestations of Alzheimer’s and Other Neurodegenerative Diseases: The Prospect of the Eye as a Tool for the Early Diagnosis of Alzheimer’s Disease. J. Ophthalmol. 2018, 2018, 8538573. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Utsunomiya, K.; Ota, H.; Ogura, Y.; Narabayashi, I.; Ikeda, T. Comparative Study of Cerebral Blood Flow in Patients With Normal-Tension Glaucoma and Control Subjects. Am. J. Ophthalmol. 2006, 141, 394–396. [Google Scholar] [CrossRef]
- Berisha, F.; Feke, G.T.; Trempe, C.L.; McMeel, J.W.; Schepens, C.L. Retinal Abnormalities in Early Alzheimer’s Disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2285–2289. [Google Scholar] [CrossRef]
- Golzan, S.M.; Goozee, K.; Georgevsky, D.; Avolio, A.; Chatterjee, P.; Shen, K.; Gupta, V.; Chung, R.; Savage, G.; Orr, C.F.; et al. Retinal Vascular and Structural Changes Are Associated with Amyloid Burden in the Elderly: Ophthalmic Biomarkers of Preclinical Alzheimer’s Disease. Alzheimer’s Res. Ther. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Nuzzi, R.; Vitale, A. Cerebral Modifications in Glaucoma and Macular Degeneration: Analysis of Current Evidence in Literature and Their Implications on Therapeutic Perspectives. Eye Brain 2021, 13, 159. [Google Scholar] [CrossRef]
- Rensma, S.P.; van Sloten, T.T.; Houben, A.J.H.M.; Köhler, S.; van Boxtel, M.P.J.; Berendschot, T.T.J.M.; Jansen, J.F.A.; Verhey, F.R.J.; Kroon, A.A.; Koster, A.; et al. Microvascular Dysfunction Is Associated With Worse Cognitive Performance: The Maastricht Study. Hypertension 2020, 75, 237–245. [Google Scholar] [CrossRef]
- Han, F. Cerebral Microvascular Dysfunction and Neurodegeneration in Dementia. Stroke Vasc. Neurol. 2019, 4, 105. [Google Scholar] [CrossRef]
- Bagi, Z.; Kroenke, C.D.; Fopiano, K.A.; Tian, Y.; Filosa, J.A.; Sherman, L.S.; Larson, E.B.; Keene, C.D.; Degener O’Brien, K.; Adeniyi, P.A.; et al. Association of Cerebral Microvascular Dysfunction and White Matter Injury in Alzheimer’s Disease. GeroScience 2022, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mudassar Imran Bukhari, S.; Yew, K.K.; Thambiraja, R.; Sulong, S.; Ghulam Rasool, A.H.; Ahmad Tajudin, L.-S. Microvascular Endothelial Function and Primary Open Angle Glaucoma. Ther. Adv. Ophthalmol. 2019, 11, 2515841419868100. [Google Scholar] [CrossRef]
- Chua, J.; Hu, Q.; Ke, M.; Tan, B.; Hong, J.; Yao, X.; Hilal, S.; Venketasubramanian, N.; Garhöfer, G.; Cheung, C.Y.; et al. Retinal Microvasculature Dysfunction Is Associated with Alzheimer’s Disease and Mild Cognitive Impairment. Alzheimer’s Res. Ther. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowska, S.; Benavente-Perez, A.; Negi, A.; Sung, V.; Patel, S.R.; Gherghel, D. Primary Open-Angle Glaucoma vs Normal-Tension Glaucoma: The Vascular Perspective. JAMA Ophthalmol. 2013, 131, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowska, S.; Ekart, A.; Sung, V.; Negi, A.; Qin, L.; Patel, S.R.; Jacob, S.; Atkins, C.; Benavente-Perez, A.; Gherghel, D. Coexistence of Macro- and Micro-Vascular Abnormalities in Newly Diagnosed Normal Tension Glaucoma Patients. Acta Ophthalmol. 2012, 90, e553–e559. [Google Scholar] [CrossRef]
- Response of Retinal Vessel Diameters to Flicker Stimulation...: Journal of Glaucoma. Available online: https://journals.lww.com/glaucomajournal/Fulltext/2004/08000/Response_of_Retinal_Vessel_Diameters_to_Flicker.13.aspx?casa_token=SKEs1Ktt_3AAAAAA:Vp7bcSc6Bi8IC4_Ksir0b0gyUs1NQ5gOxnki43o73vogt2m3Iq_YxwioxOYyrqgw0Xv73yX7NqWSsfoVFD0m_ehaFAE (accessed on 25 May 2021).
- Mroczkowska, S.; Benavente-Perez, A.; Patel, S.; Qin, L.; Bentham, P.; Gherghel, D. Retinal Vascular Dysfunction Relates to Cognitive Impairment in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2014, 28, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M.; Suwala, K.; Zabel, K.; Zaron, A.; Kucharski, R.; Araszkiewicz, A. Comparison of Retinal Microvasculature in Patients With Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3447–3455. [Google Scholar] [CrossRef]
- Mills, R.P.; Budenz, D.L.; Lee, P.P.; Noecker, R.J.; Walt, J.G.; Siegartel, L.R.; Evans, S.J.; Doyle, J.J. Categorizing the Stage of Glaucoma From Pre-Diagnosis to End-Stage Disease. Am. J. Ophthalmol. 2006, 141, 24–30. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- A Practical Method for Grading the Cognitive State of Patients for the Clinician | CiNii Research. Available online: https://cir.nii.ac.jp/crid/1571417125069760128 (accessed on 1 August 2022).
- Gould, N. Guidelines across the Health and Social Care Divides: The Example of the NICE-SCIE Dementia Guideline. Int. Rev. Psychiatry 2011, 23, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A Brief Cognitive Test Battery for Dementia Screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Chylack, L.T.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Azizi, B.; Wong, T.; Wan, J.; Singer, S.; Hudson, C. The Impact of Cataract on the Quantitative, Non-Invasive Assessment of Retinal Blood Flow. Acta Ophthalmol. 2012, 90, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Karimzad, S.; Bilkhu, P.S.; Wolffsohn, J.S.; Bellary, S.; Shokr, H.; Singhal, R.; Gherghel, D. Impact of Bariatric Surgery-Induced Weight Loss on Anterior Eye Health in Patients with Obesity. Nutrients 2022, 14, 2462. [Google Scholar] [CrossRef]
- Shokr, H.; Wolffsohn, J.S.; Trave Huarte, S.; Scarpello, E.; Gherghel, D.; Ophthalmol, A. Dry Eye Disease Is Associated with Retinal Microvascular Dysfunction and Possible Risk for Cardiovascular Disease. Acta Ophthalmol. 2021, 99, aos.14782. [Google Scholar] [CrossRef]
- Shokr, H.; Gherghel, D. European Society of Cardiology/European Society of Hypertension versus the American College of Cardiology/American Heart Association Guidelines on the Cut-off Values for Early Hypertension: A Microvascular Perspective. Sci. Rep. 2021, 11, 3473. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Shokr, H.; Dias, I.H.K.; Gherghel, D. Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants 2021, 10, 1756. [Google Scholar] [CrossRef]
- Nagel, E.; Vilser, W. Flicker Observation Light Induces Diameter Response in Retinal Arterioles: A Clinical Methodological Study. Br. J. Ophthalmol. 2004, 88, 54–56. [Google Scholar] [CrossRef]
- Shokr, H.; Dias, I.H.K.; Gherghel, D. Microvascular Function and Oxidative Stress in Adult Individuals with Early Onset of Cardiovascular Disease. Sci. Rep. 2020, 10, 4881. [Google Scholar] [CrossRef]
- Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal and Peripheral Vascular Function in Healthy Individuals with Low Cardiovascular Risk. Microvasc. Res. 2019, 126, 103908. [Google Scholar] [CrossRef]
- Shokr, H.; Lush, V.; Dias, I.H.; Ekárt, A.; De Moraes, G.; Gherghel, D. The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk. Cells 2022, 11, 3037. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal Vascular Function in Asymptomatic Individuals with a Positive Family History of Cardiovascular Disease. Acta Ophthalmol. 2018, 96, e956–e962. [Google Scholar] [CrossRef] [PubMed]
- Tutaj, M.; Brown, C.M.; Brys, M.; Marthol, H.; Hecht, M.J.; Dutsch, M.; Michelson, G.; Hilz, M.J. Dynamic Cerebral Autoregulation Is Impaired in Glaucoma. J. Neurol. Sci. 2004, 220, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Levi, C.R.; Schofield, P.; Wang, Y.; Lovett, E.C. Quantitative Measurement of Cerebral Haemodynamics in Early Vascular Dementia and Alzheimer’s Disease. J. Clin. Neurosci. 2006, 13, 563–568. [Google Scholar] [CrossRef]
- Marchesi, V.T. Alzheimer’s Dementia Begins as a Disease of Small Blood Vessels, Damaged by Oxidative-Induced Inflammation and Dysregulated Amyloid Metabolism: Implications for Early Detection and Therapy. FASEB J. 2011, 25, 5–13. [Google Scholar] [CrossRef]
- Prada, D.; Harris, A.; Guidoboni, G.; Siesky, B.; Huang, A.M.; Arciero, J. Autoregulation and Neurovascular Coupling in the Optic Nerve Head. Surv. Ophthalmol. 2016, 61, 164–186. [Google Scholar] [CrossRef]
- Conzen, C.; Albanna, W.; Weiss, M.; Kürten, D.; Vilser, W.; Kotliar, K.; Zäske, C.; Clusmann, H.; Schubert, G.A. Vasoconstriction and Impairment of Neurovascular Coupling after Subarachnoid Hemorrhage: A Descriptive Analysis of Retinal Changes. Transl. Stroke Res. 2018, 9, 284–293. [Google Scholar] [CrossRef]
- Lipecz, A.; Csipo, T.; Tarantini, S.; Hand, R.A.; Ngo, B.T.N.; Conley, S.; Nemeth, G.; Tsorbatzoglou, A.; Courtney, D.L.; Yabluchanska, V.; et al. Age-Related Impairment of Neurovascular Coupling Responses: A Dynamic Vessel Analysis (DVA)-Based Approach to Measure Decreased Flicker Light Stimulus-Induced Retinal Arteriolar Dilation in Healthy Older Adults. GeroScience 2019, 41, 341–349. [Google Scholar] [CrossRef]
- Albanna, W.; Kotliar, K.; Luke, J.N.; Alpdogan, S.; Conzen, C.; Lindauer, U.; Clusmann, H.; Hescheler, J.; Vilser, W.; Schneider, T.; et al. Non-Invasive Evaluation of Neurovascular Coupling in the Murine Retina by Dynamic Retinal Vessel Analysis. PLoS ONE 2018, 13, e0204689. [Google Scholar] [CrossRef]
- Dorner, G.T.; Garhofer, G.; Kiss, B.; Polska, E.; Polak, K.; Riva, C.E.; Schmetterer, L. Nitric Oxide Regulates Retinal Vascular Tone in Humans. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 631–636. [Google Scholar] [CrossRef]
- Polak, K.; Luksch, A.; Berisha, F.; Fuchsjaeger-Mayrl, G.; Dallinger, S.; Schmetterer, L. Altered Nitric Oxide System in Patients With Open-Angle Glaucoma. Arch. Ophthalmol. 2007, 125, 494–498. [Google Scholar] [CrossRef]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The Nitric Oxide-Guanylate Cyclase Pathway and Glaucoma. Nitric Oxide 2018, 77, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of Endothelial Dysfunction in the Development of Alzheimer’s Disease: Is Alzheimer’s a Vascular Disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197. [Google Scholar]
- Alzheimer´s Disease and Oxidative Stress: A Review: Ingenta Connect. Available online: https://www.ingentaconnect.com/content/ben/cmc/2014/00000021/00000003/art00007 (accessed on 4 August 2022).
- Prasanna, G.; Krishnamoorthy, R.; Yorio, T. Endothelin, Astrocytes and Glaucoma. Exp. Eye Res. 2011, 93, 170–177. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; Slaoui, L.; Mazaré, N.; Gilbert, A.; Oudart, M.; Alvear-Perez, R.; Elorza-Vidal, X.; Chever, O.; Boulay, A.C. Astrocytes in the Regulation of Cerebrovascular Functions. Glia 2021, 69, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Heinecke, J.W. Oxidative Stress and Endothelial Dysfunction in Vascular Disease. Curr. Diabetes Rep. 2007, 7, 257–264. [Google Scholar] [CrossRef]
- Ruan, Y.; Patzak, A.; Pfeiffer, N.; Gericke, A. Muscarinic Acetylcholine Receptors in the Retina—Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4989. [Google Scholar] [CrossRef] [PubMed]
- Berra, A.; Ganzinelli, S.; Saravia, M.; Borda, E.; Sterin-Borda, L. Inducible Nitric Oxide Synthase Subserves Cholinergic Vasodilation in Retina. Vis. Neurosci. 2005, 22, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.T.; Louzada, P.R.; De Mello, F.G.; Ferreira, S.T. Amyloid-β Decreases Nitric Oxide Production in Cultured Retinal Neurons: A Possible Mechanism for Synaptic Dysfunction in Alzheimer’s Disease? Neurochem. Res. 2011, 36, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, Y.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Rundek, T.; Baumel, B.S.; Landman, J.; Wang, J. Impaired Retinal Microcirculation in Patients with Alzheimer’s Disease. PLoS ONE 2018, 13, e0192154. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Lucio, M.; Schlick, S.; Wollborn, A.; Hosari, S.; Mardin, C. OCT-Angiography: Regional Reduced Macula Microcirculation in Ocular Hypertensive and Pre-Perimetric Glaucoma Patients. PLoS ONE 2021, 16, e0246469. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Konieczka, K.; Liu, X.; Chen, M.; Yao, K.; Wang, K.; Flammer, J. Role of Ocular Blood Flow in Normal Tension Glaucoma. Adv. Ophthalmol. Pract. Res. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Killer, H.; Pircher, A. Normal Tension Glaucoma: Review of Current Understanding and Mechanisms of the Pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Chakraborty, A. Vascular Involvement in Alzheimer’s Disease from Bench to Bedside. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2020. [Google Scholar]
| AD (1) | POAG (2) | Controls (3) | ANOVA p-Value | Significance | |
|---|---|---|---|---|---|
| N | 10 | 19 | 20 | - | - |
| Gender | 5F:5M | 9F:10M | 8F:12M | 0.737 | - |
| Age (years) | 62.50 ± 8.07 | 63.93 ± 8.26 | 58.00 ± 4.32 | 0.079 | - |
| SBP (mmHg) | 141.70 ± 14.21 | 135.50 ± 16.92 | 131.70 ± 17.90 | 0.318 | - |
| DBP (mmHg) | 80.30 ± 7.51 | 78.86 ± 10.40 | 79.70 ± 9.39 | 0.930 | - |
| BMI | 27.61 ± 5.80 | 27.36 ± 4.03 | 27.56 ± 4.67 | 0.990 | - |
| Glucose | 4.40 ± 1.44 | 4.44 ± 1.03 | 4.87 ± 1.02 | 0.469 | - |
| TG | 1.28 ± 0.60 | 1.09 ± 0.35 | 1.17 ± 0.40 | 0.575 | - |
| HDL-C (mmol/L) | 1.33 ± 0.25 | 1.23 ± 0.24 | 1.14 ± 0.32 | 0.245 | - |
| Total-C (mmol/L) | 4.77 ± 0.64 | 4.20 ± 0.84 | 4.73 ± 0.67 | 0.089 | - |
| LDL-C | 3.18 ± 0.67 | 2.87 ± 0.75 | 3.38 ± 0.74 | 0.368 | |
| IOP (mmHg) | 16.50 ± 2.12 | 23.25 ± 2.38 | 17.20 ± 2.68 | <0.001 * | 2 > 1, 3; 1 = 3 |
| OPP | 84.96 ± 9.46 | 47.12 ± 16.93 | 82.65 ± 12.06 | <0.001 * | 2 < 1, 3; 1 = 3 |
| ARTERY Average Data | AD (1) | POAG (2) | Controls (3) | p-Value |
|---|---|---|---|---|
| MD (%) | 5.53 ± 3.25 | 5.75 ± 3.57 | 5.19 ± 2.19 | 0.853 |
| RT | 24.04 ± 11.74 | 21.58 ± 6.89 | 20.48 ± 6.77 | 0.442 |
| BFR | 3.39 ± 3.79 | 2.55 ± 4.58 | 2.80 ± 1.89 | 0.832 |
| MC (%) | −3.23 ± 1.56 | −4.58 ± 4.58 | −2.55 ± 1.85 | 0.100 |
| tMC (secs) | 29.00 ± 10.24 | 33.47 ± 10.07 | 26.43 ± 8.29 | 0.100 |
| ARTERY | AD (1) | POAG (2) | Controls (3) | p-Value | Significance | Between Groups p-Value |
|---|---|---|---|---|---|---|
| RT | ||||||
| Flicker 1 | 29.30 ± 16.61 | 18.67 ± 13.16 | 20.05 ± 12.07 | 0.134 | ||
| Flicker 2 | 16.30 ± 11.48 | 20.60 ± 12.12 | 25.85 ± 9.00 | 0.068 | ||
| Flicker 3 | 27.89 ± 17.62 | 27.92 ± 10.14 | 15.55 ± 11.07 | 0.009 * | 1, 2 > 3 | |
| Within groups ANOVA | 0.093 | 0.067 | 0.011 * | 0.001 * |
| VEIN | AD (1) | POAG (2) | Controls (4) | ANOVA p-Value |
|---|---|---|---|---|
| MD (%) | 6.12 ± 3.14 | 5.24 ± 1.53 | 5.13 ± 2.86 | 0.614 |
| RT | 22.67 ± 9.39 | 19.69 ± 4.24 | 20.48 ± 3.68 | 0.430 |
| BFR | 2.16 ± 4.03 | 2.83 ± 2.54 | 3.30 ± 2.28 | 0.595 |
| MC (%) | −2.61 ± 2.13 | −2.54 ± 2.45 | −1.77 ± 1.40 | 0.422 |
| tMC (secs) | 29.74 ± 6.20 | 34.10 ± 8.40 | 34.25 ± 9.40 | 0.443 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczkowska, S.; Shokr, H.; Benavente-Pérez, A.; Negi, A.; Bentham, P.; Gherghel, D. Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients. J. Clin. Med. 2022, 11, 6702. https://doi.org/10.3390/jcm11226702
Mroczkowska S, Shokr H, Benavente-Pérez A, Negi A, Bentham P, Gherghel D. Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients. Journal of Clinical Medicine. 2022; 11(22):6702. https://doi.org/10.3390/jcm11226702
Chicago/Turabian StyleMroczkowska, Stephanie, Hala Shokr, Alexandra Benavente-Pérez, Anil Negi, Peter Bentham, and Doina Gherghel. 2022. "Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients" Journal of Clinical Medicine 11, no. 22: 6702. https://doi.org/10.3390/jcm11226702
APA StyleMroczkowska, S., Shokr, H., Benavente-Pérez, A., Negi, A., Bentham, P., & Gherghel, D. (2022). Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients. Journal of Clinical Medicine, 11(22), 6702. https://doi.org/10.3390/jcm11226702







