Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Interventions
2.3. Measurements: Dual Energy X-ray Absorptiometry (DXA)
2.4. Magnetic Resonance Imaging (MRI)
2.5. Modified Ashworth Scale and Penn Spasms Frequency Scale
2.6. Isometric Peak Torque
2.7. Statistical Analysis
3. Results
3.1. NMES-Amplitude and Repetitions
3.2. Modified Ashworth Scale and Penn Spasms Frequency Scale
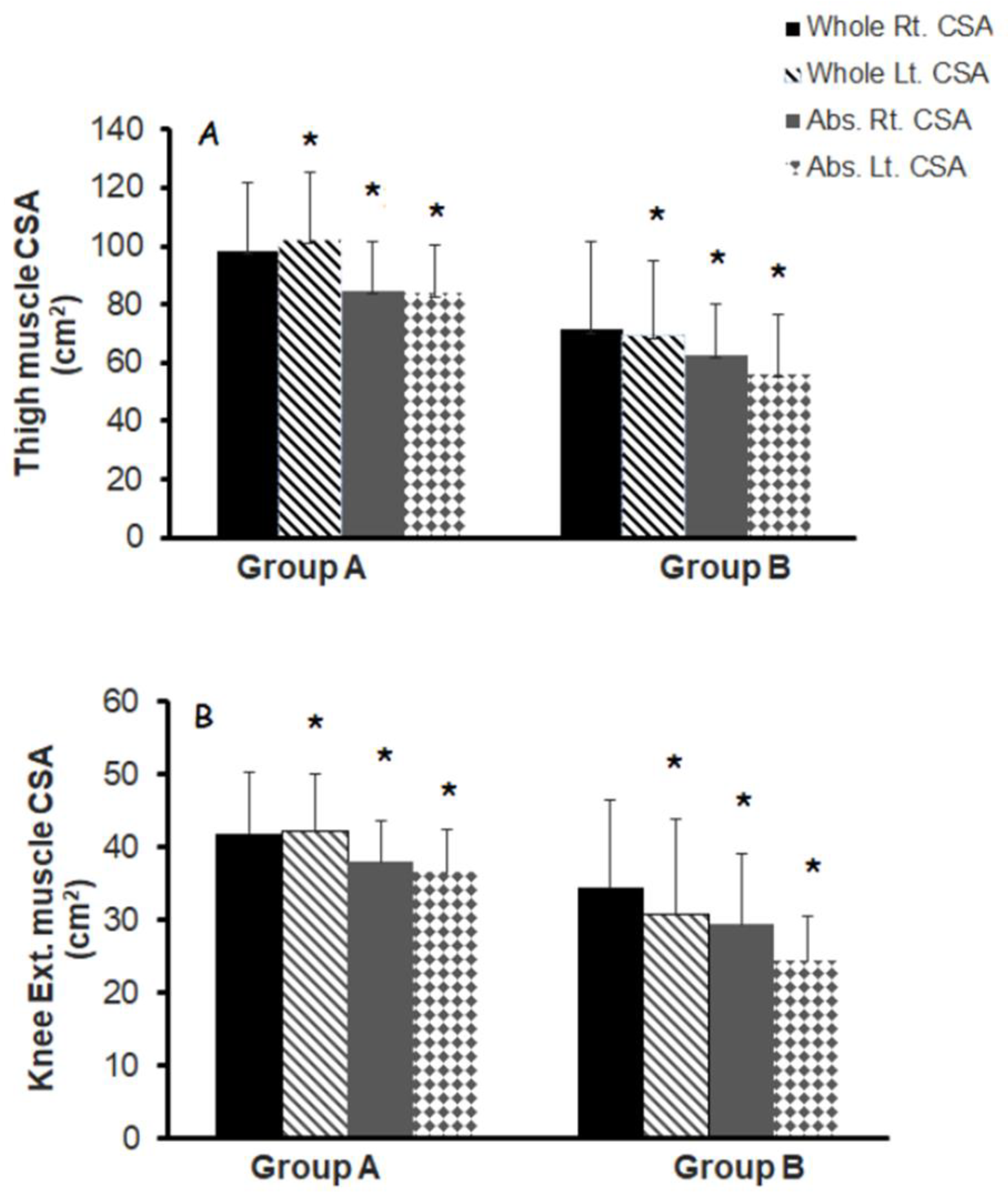
3.3. Muscle Quality
3.4. Isometric Knee Extensor Peak Torque
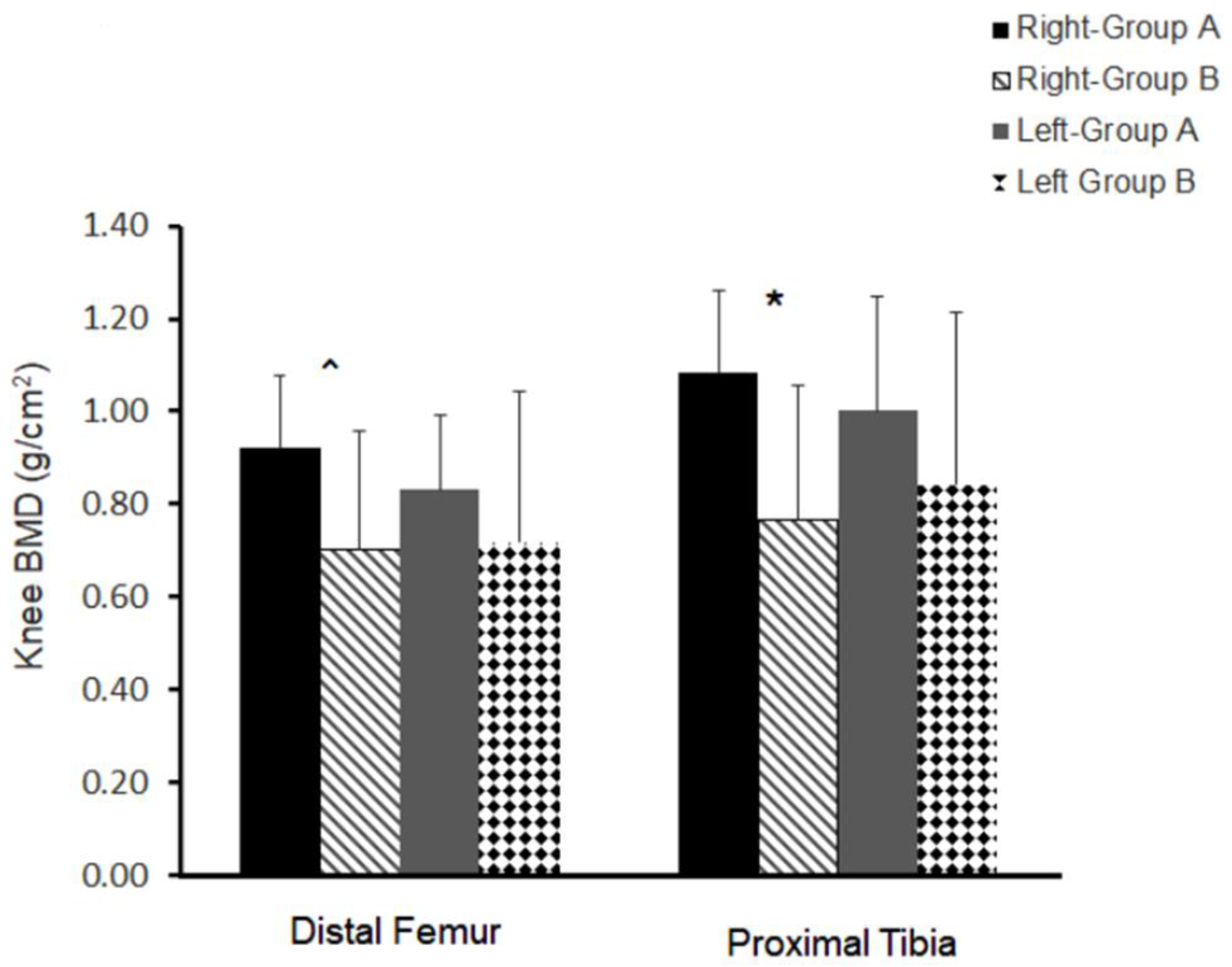
3.5. Bone Quality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Hachmann, J.T.; Yousak, A.; Wallner, J.J.; Gad, P.N.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, 126, 1843–1859. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Gill, S.; Holman, M.E.; Davis, J.C.; Atri, R.; Bai, O.; Goetz, L.; Lester, D.L.; Trainer, R.; Lavis, T.D. The feasibility of using exoskeletal-assisted walking with epidural stimulation: A case report study. Ann. Clin. Transl. Neurol. 2020, 7, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.J.; Apple, D.F., Jr.; Hillegass, E.A.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 373–378. [Google Scholar] [CrossRef]
- Spungen, A.M.; Adkins, R.H.; Stewart, C.A.; Wang, J.; Pierson, R.N., Jr.; Waters, R.L.; Bauman, W.A. Factors influencing body composition in persons with spinal cord injury: A cross-sectional study. J. Appl. Physiol. 2003, 95, 2398–2407. [Google Scholar] [CrossRef]
- Elder, C.P.; Apple, D.F.; Bickel, C.S.; A Meyer, R.; Dudley, G.A. Intramuscular fat and glucose tolerance after spinal cord injury—A cross-sectional study. Spinal Cord 2004, 42, 711–716. [Google Scholar] [CrossRef]
- Ogawa, M.; Lester, R.; Akima, H.; Gorgey, A.S. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen. Res. 2017, 12, 2100–2105. [Google Scholar]
- Moore, C.D.; Craven, B.; Thabane, L.; Laing, A.; Frank-Wilson, A.; Kontulainen, S.; Papaioannou, A.; Adachi, J.; Giangregorio, L. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 2015, 15, 32–41. [Google Scholar]
- Ghatas, M.P.; Khan, M.R.; Gorgey, A.S. Skeletal muscle stiffness as measured by magnetic resonance elastography after chronic spinal cord injury: A cross-sectional pilot study. Neural Regen. Res. 2021, 16, 2486–2493. [Google Scholar]
- Holman, M.; Chang, G.; Ghatas, M.; Saha, P.; Zhang, X.; Khan, M.; Sima, A.; Adler, R.; Gorgey, A.S. Bone and non-contractile soft tissue changes following open kinetic chain resistance training and testosterone treatment in spinal cord injury: An exploratory study. Osteoporos. Int. 2021, 32, 1321–1332. [Google Scholar] [CrossRef]
- Holman, M.; Gorgey, A. Testosterone and Resistance Training Improve Muscle Quality in Spinal Cord Injury. Med. Sci. Sports Exerc. 2019, 51, 1591–1598. [Google Scholar] [CrossRef]
- Cirnigliaro, C.M.; Myslinski, M.J.; La Fountaine, M.F.; Kirshblum, S.C.; Forrest, G.F.; Bauman, W.A. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: Imaging approaches, risk of fracture, and potential treatment options. Osteoporos. Int. 2017, 28, 747–765. [Google Scholar] [CrossRef]
- Qin, W.; Bauman, W.A.; Cardozo, C. Bone and muscle loss after spinal cord injury: Organ interactions. Ann. N. Y. Acad. Sci. 2010, 1211, 66–84. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Daniels, J.A.; Adler, R.A.; Moore, J.R.; Gater, D.R., Jr. The effects of spinal cord injury and exercise on bone mass: A literature review. NeuroRehabilitation 2011, 29, 261–269. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H.; Kushwaha, V.; Stewart, C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J. Spinal Cord Med. 2004, 27, 202–206. [Google Scholar] [CrossRef]
- Biering-Sørensen, F.; Bohr, H.H.; Schaadt, O.P. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur. J. Clin. Investig. 1990, 20, 330–335. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cirnigliaro, C.M.; La Fountaine, M.F.; Martinez, L.; Kirshblum, S.C.; Spungen, A.M. Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: An observational cohort study. J. Bone Miner. Metab. 2015, 33, 410–421. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H.; A Stewart, C. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J. Spinal Cord Med. 2008, 31, 543–550. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Poarch, H.J.; Adler, R.A.; Khalil, R.E.; Gater, D.R. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM&R 2013, 5, 939–948. [Google Scholar]
- Modlesky, C.M.; Slade, J.M.; Bickel, C.S.; Meyer, R.A.; Dudley, G. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone 2005, 36, 331–339. [Google Scholar] [CrossRef]
- Liu, C.C.; Theodorou, D.J.; Andre, M.P.; Sartoris, D.J.; Szollar, S.M.; Martin, E.M.; Deftos, L.J.; Theodorou, S.J. Quantitative computed tomography in the evaluation of spinal osteoporosis following spinal cord injury. Osteoporos. Int. 2000, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Ghatas, M.P.; Sutor, T.W.; Gorgey, A.S. Prediction of Distal Femur and Proximal Tibia Bone Mineral Density from Total Body Dual Energy X-ray Absorptiometry Scans in Persons with Spinal Cord Injury. J. Clin. Densitom. 2022, 25, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Cho, G.M.; Dolbow, D.R.; Gater, D.R. Differences in current amplitude evoking leg extension in individuals with spinal cord injury. NeuroRehabilitation 2013, 33, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Khalil, R.E.; Davis, J.C.; Carter, W.; Gill, R.; Rivers, J.; Khan, R.; Goetz, L.L.; Castillo, T.; Lavis, T.; et al. Skeletal muscle hypertrophy and attenuation of cardio-metabolic risk factors (SHARC) using functional electrical stimulation-lower extremity cycling in persons with spinal cord injury: Study protocol for a randomized clinical trial. Trials 2019, 20, 526. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.S.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Krueger, E.; Popović-Maneski, L.; Neto, G.N.N.; Scheeren, E.M.; Fiusa, J.M.; Nohama, P. Neuromuscular fatigue detection by mechanomyography in people with complete spinal cord injury. Res. Biomed. Eng. 2020, 36, 203–212. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Cirnigliaro, C.M.; Bauman, W.A.; Adler, R.A. Estimates of the precision of regional and whole body composition by dual-energy X-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord 2018, 56, 987–995. [Google Scholar] [CrossRef]
- Gorgey, A.; Lester, R.; Johnson, K.; Khalil, R.; Khan, R. MRI analysis and clinical significance of lower extremity muscle cross-sectional area after spinal cord injury. Neural Regen. Res. 2017, 12, 714–722. [Google Scholar] [CrossRef]
- Wade, R.C.; Lester, R.M.; Gorgey, A.S. Validation of Anthropometric Muscle Cross-Sectional Area Equation after Spinal Cord Injury. Int. J. Sports Med. 2018, 39, 366–373. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Chiodo, A.E.; Zemper, E.D.; Hornyak, J.E.; Rodriguez, G.M.; Gater, D.R. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J. Spinal Cord Med. 2010, 33, 6–15. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dudley, G.A. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord 2008, 46, 96–102. [Google Scholar] [CrossRef]
- Mills, P.B.; Vakil, A.P.; Phillips, C.; Kei, L.; Kwon, B.K. Intra-rater and inter-rater reliability of the Penn Spasm Frequency Scale in People with chronic traumatic spinal cord injury. Spinal Cord 2018, 56, 569–574. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Gater, D.R. Insulin growth factors may explain relationship between spasticity and skeletal muscle size in men with spinal cord injury. J. Rehabil. Res. Dev. 2012, 49, 373–380. [Google Scholar] [CrossRef]
- Bickel, C.S.; Slade, J.; Mahoney, E.; Haddad, F.; Dudley, G.A.; Adams, G.R. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J. Appl. Physiol. 2005, 98, 482–488. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar]
- Lofvenmark, I.; Werhagen, L.; Norrbrink, C. Spasticity and bone density after a spinal cord injury. J. Rehabil. Med. 2009, 41, 1080–1084. [Google Scholar] [CrossRef]
- Ifon, D.E.; Ghatas, M.P.; Davis, J.C.; E Khalil, R.; Adler, R.A.; Gorgey, A.S. Long-term effect of intrathecal baclofen treatment on bone health and body composition after spinal cord injury: A case matched report. World J. Orthop. 2020, 11, 453–464. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brizendine, J.T.; Backus, D.; McCully, K.K. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 2166–2173. [Google Scholar] [CrossRef]
- Modlesky, C.M.; Majumdar, S.; Narasimhan, A.; Dudley, G.A. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J. Bone Miner. Res. 2004, 19, 48–55. [Google Scholar] [CrossRef]
- Morse, L.R.; Biering-Soerensen, F.; Carbone, L.D.; Cervinka, T.; Cirnigliaro, C.M.; Johnston, T.E.; Liu, N.; Troy, K.; Weaver, F.M.; Shuhart, C.; et al. Bone Mineral Density Testing in Spinal Cord Injury: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Spungen, A.M.; Bauman, W.A.; Biswas, K.; Jones, K.M.; Snodgrass, A.J.; Goetz, L.L.; Gorman, P.H.; Kirshblum, S.; Sabharwal, S.; White, K.T.; et al. The design of a randomized control trial of exoskeletal-assisted walking in the home and community on quality of life in persons with chronic spinal cord injury. Contemp. Clin. Trials 2020, 96, 106102. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Goldsmith, J.A.; Khalil, R.E.; Liu, X.-H.; Pan, J.; Cardozo, C.; Adler, R.A. Predictors of muscle hypertrophy responsiveness to electrically evoked resistance training after spinal cord injury. Eur. J. Appl. Physiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Group A (n = 8) | Group B (n = 9) | Between Group p-Values |
|---|---|---|---|
| Age (years) | 38 ± 12 | 37 ± 13 | 0.88 |
| Ethnicity | African American (n = 3) White (n = 5) | African American (n = 4) White (n = 5) | - |
| Gender | 7 male, 1 female | 8 male, 1 female | |
| Weight (kg) | 74 ± 14 | 69 ± 21 | 0.51 |
| Height (m) | 1.75 ± 1.0 | 1.74 ± 0.7 | 0.7 |
| BMI (kg/m2) | 24.3 ± 5.4 | 22.7 ± 6.8 | 0.58 |
| Paraplegia/Tetraplegia | 7/1 | 4/5 | - |
| Single Neurological Level (SNL) | C5-T12 | C6-T12 | - |
| TSI (years) | 12.2 ± 11.0 | 12.0 ± 11.0 | 0.96 |
| ISNCSCI classification | A (n = 2) B (n = 4) | A (n = 7) B (n = 1) | - |
| C (n = 2) | C (n = 1) |
| Characteristics | Group A (n = 8) | Group B (n = 9) | p-Values | |
|---|---|---|---|---|
| Hip MAS | Flexors | 1 ± 1.07 | 0.77 ± 0.64 | 0.64 |
| Knee MAS | Extensors Flexors | 1 ± 1.1 1.12 ± 1.25 | 0.77 ± 0.97 0.44 ± 0.52 | 0.68 0.18 |
| Extensors | 1.75 ± 1.0 | 0.77 ± 0.83 | 0.053 | |
| Ankle MAS | Dorsiflexors | 0.75 ± 0.89 | 0.33 ± 0.50 | 0.26 |
| Planter flexors | 1.12 ± 0.99 | 0.22 ± 0.44 | 0.04 | |
| Penn Spasm Frequency Scale | 2.9 ± 0.83 | 1.1 ± 1.16 | 0.002 | |
| Amplitude of the current—Week 1 (mA) | Sets 1–2 (R/L) Set 3–4 (R/L) | 73 ± 14/70 ± 15 80 ± 15/76 ± 20 | 137 ± 33/131 ± 33 148 ± 36/145 ± 32 | 0.0002/0.0008 0.0003/0.0002 |
| Repetitions | Week 1 (R/L) | 75 ± 14/75 ± 13 | 50 ± 22/55 ± 21 | 0.01/0.03 |
| Weights (lbs.) | Week 1 | 0/0 | 0/0 | |
| Week 2 (mA) | Sets 1–2 (R/L) Set 3–4 (R/L) | 77 ± 17/70 ± 13 84 ± 19/77 ± 18 | 126 ± 37/125 ± 21 136 ± 34/137 ± 25 | 0.004/0.0003 0.001/0.0003 |
| Repetitions | Week 2 (R/L) | 80 ± 0/80 ± 0 | 56 ± 17/64 ± 24 | 0.002/0.07 |
| Weights (lbs.) | Week 2 | 2 ± 0/1.8 ± 0.7 | 0.2 ± 0.7/0.3 ± 0.7 | 0.0001/0.0004 |
| Week 3 (mA) | Sets 1–2 (R/L) Set 3–4 (R/L) | 78 ± 18/77 ± 17 85 ± 22/85 ± 19 | 124 ± 33/123 ± 30 132 ± 33/134 ± 32 | 0.003/0.005 0.003/0.004 |
| Repetitions | Week 3 (R/L) | 70 ± 19/70 ± 16 | 61 ± 18/64 ± 21 | 0.3/0.4 |
| Weights (lbs.) | Week 3 | 4 ± 0/3.2 ± 1.7 | 0.4 ± 0.8/0.9 ± 1.0 | 0.0001/0.0002 |
| Dependent Variables | Independent Variables (Predictors) | R2 | p-Values | |
|---|---|---|---|---|
| Model 1 | Whole thigh muscle CSA (cm2) | 1.3 × body weight + 0.054 × amplitude of the current + 0.684 × reps-58.7 | 0.92 | 0.001 |
| Model 2 | Absolute whole thigh muscle CSA (cm2) | 0.94 × body weight + 0.027 × amplitude of the current + 0.612 × reps-38.4 | 0.87 | 0.001 |
| Model 3 | Knee extensors muscle CSA (cm2) | 0.499 × body weight + 0.045 × amplitude of the current + 0.301 × reps-23 | 0.88 | 0.001 |
| Model 4 | Absolute knee extensors muscle CSA (cm2) | 0.401 × body weight + 0.031 × amplitude of the current + 0.276 × reps-17.3 | 0.84 | 0.001 |
| Model 5 | IMF-whole thigh CSA (cm2) | 0.37 × body weight + 0.027 × amplitude of the current + 0.072 × reps-20.3 | 0.55 | 0.026 |
| Model 6 | Distal femur BMD (g/cm2) | 0.006 × body weight + 0.002 × amplitude of the current + 0.006 × reps-0.248 | 0.67 | 0.016 |
| Model 7 | Proximal tibia BMD (g/cm2) | 0.010 × body weight + 0.001 × amplitude of the current + 0.07 × reps-0.39 | 0.71 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorgey, A.S.; Khalil, R.E.; Sutor, T.W.; Goldsmith, J.A.; Cifu, D.X. Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. J. Clin. Med. 2022, 11, 6681. https://doi.org/10.3390/jcm11226681
Gorgey AS, Khalil RE, Sutor TW, Goldsmith JA, Cifu DX. Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. Journal of Clinical Medicine. 2022; 11(22):6681. https://doi.org/10.3390/jcm11226681
Chicago/Turabian StyleGorgey, Ashraf S., Refka E. Khalil, Tommy W. Sutor, Jacob A. Goldsmith, and David X. Cifu. 2022. "Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury" Journal of Clinical Medicine 11, no. 22: 6681. https://doi.org/10.3390/jcm11226681
APA StyleGorgey, A. S., Khalil, R. E., Sutor, T. W., Goldsmith, J. A., & Cifu, D. X. (2022). Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. Journal of Clinical Medicine, 11(22), 6681. https://doi.org/10.3390/jcm11226681







