Patients with Schizophrenia Showed Worse Cognitive Performance than Bipolar and Major Depressive Disorder in a Sample with Comorbid Substance Use Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Instruments
2.2.1. Sociodemographic and Clinical Measures
2.2.2. Neurocognitive Performance Assessment Battery
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic Data and Clinical Variables Related to the Comorbid Severe Mental Illness
3.2. Comparisons of Neurocognitive Performance among the Groups and Considering Normative Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keen, C.; Kinner, S.A.; Young, J.T.; Jang, K.; Gan, W.; Samji, H.; Zhao, B.; Krausz, M.; Slaunwhite, A. Prevalence of co-occurring mental illness and substance use disorder and association with overdose: A linked data cohort study among residents of British Columbia, Canada. Addiction 2022, 117, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, A.; Waqas, A.; Naveed, S.; Hossain, M.M.; Rahman, A.; Saeed, K.; Fuhr, D.C. Prevalence of mental disorders in the WHO eastern mediterranean region: A systematic review and meta-analysis. Front. Psychiatry 2021, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.E.; Malhi, G.S.; Lai, H.M.X.; Cleary, M. Prevalence of comorbid substance use in major depressive disorder in community and clinical settings, 1990–2019: Systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Arnau, F.; Benito, A.; Villar, M.; Ortega, M.E.; López-Peláez, L.; Haro, G. Addressing dual disorders in a medium-term admission unit. Brain Sci. 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.E.; Large, M.M.; Cleary, M.; Lai, H.M.X.; Saunders, J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018, 191, 234–258. [Google Scholar] [CrossRef]
- Lai, H.M.X.; Cleary, M.; Sitharthan, T.; Hunt, G.E. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Almeida, P.P.; de Araujo Filho, G.M.; Malta, S.M.; Laranjeira, R.R.; Marques, A.C.R.P.; Bressan, R.A.; Lacerda, A.L.T. Attention and memory deficits in crack-cocaine users persist over four weeks of abstinence. J. Subst. Abuse Treat. 2017, 81, 73–78. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Garcia-Fernandez, G.; Dom, G. Cognition and addiction. Dialogues Clin. Neurosci. 2019, 21, 281–290. [Google Scholar] [CrossRef]
- Capella, M.D.M.; Benaiges, I.; Adan, A. Neuropsychological performance in polyconsumer men under treatment. Influence of age of onset of substance use. Sci. Rep. 2015, 5, 12038. [Google Scholar] [CrossRef]
- Scott, J.C.; Lynch, K.G.; Cenkner, D.P.; Kehle-Forbes, S.M.; Polusny, M.A.; Gur, R.C.; Chen, S.; Foa, E.B.; Oslin, D.W. Neurocognitive predictors of treatment outcomes in psychotherapy for comorbid PTSD and substance use disorders. J. Consult. Clin. Psychol. 2021, 89, 937–946. [Google Scholar] [CrossRef]
- Rezapour, T.; DeVito, E.E.; Sofuoglu, M.; Ekhtiari, H. Perspectives on neurocognitive rehabilitation as an adjunct treatment for addictive disorders: From cognitive improvement to relapse prevention. In Progress in Brain Research; Ekhtiari, H., Paulus, M.P., Eds.; Elsevier: Cham, Switzerland, 2016; Volume 224, pp. 345–369. [Google Scholar]
- Scott, T.M.; Arnsten, J.; Olsen, J.P.; Arias, F.; Cunningham, C.O.; Rivera Mindt, M. Neurocognitive, psychiatric, and substance use characteristics in a diverse sample of persons with OUD who are starting methadone or buprenorphine/naloxone in opioid treatment programs. Addict. Sci. Clin. Pract. 2021, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Castine, B.R.; Albein-Urios, N.; Lozano-Rojas, O.; Martinez-Gonzalez, J.M.; Hohwy, J.; Verdejo-Garcia, A. Self-awareness deficits associated with lower treatment motivation in cocaine addiction. Am. J. Drug Alcohol Abuse 2019, 45, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Arredondo, A.Y.; Capella, M.D.M.; Prat, G.; Forero, D.A.; Navarro, J.F. Neurobiological underpinnings and modulating factors in schizophrenia spectrum disorders with a comorbid substance use disorder: A systematic review. Neurosci. Biobehav. Rev. 2017, 75, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Balanza-Martinez, V.; Crespo-Facorro, B.; Gonzalez-Pinto, A.; Vieta, E. Bipolar disorder comorbid with alcohol use disorder: Focus on neurocognitive correlates. Front. Physiol. 2015, 6, 108. [Google Scholar] [CrossRef]
- Hunt, S.A.; Kay-Lambkin, F.J.; Baker, A.L.; Michie, P.T. Systematic review of neurocognition in people with co-occurring alcohol misuse and depression. J. Affect. Disord. 2015, 179, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Scholes, K.E.; Martin-Iverson, M.T. Cannabis use and neuropsychological performance in healthy individuals and patients with schizophrenia. Psychol. Med. 2010, 40, 1635–1646. [Google Scholar] [CrossRef]
- Schnakenberg Martin, A.M.; Bonfils, K.A.; Davis, B.J.; Smith, E.A.; Schuder, K.; Lysaker, P.H. Compared to high and low cannabis use, moderate use is associated with fewer cognitive deficits in psychosis. Schizophr. Res. Cogn. 2016, 6, 15–21. [Google Scholar] [CrossRef]
- Schnell, T.; Koethe, D.; Daumann, J.; Gouzoulis-Mayfrank, E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology 2009, 205, 45–52. [Google Scholar] [CrossRef]
- Lepage, M.; Bodnar, M.; Bowie, C.R. Neurocognition: Clinical and functional outcomes in schizophrenia. Can. J. Psychiatry 2014, 59, 5–12. [Google Scholar] [CrossRef]
- Epstein, K.A.; Kumra, S. Executive attention impairment in adolescents with schizophrenia who have used cannabis. Schizophr. Res. 2014, 157, 48–54. [Google Scholar] [CrossRef]
- Shah, R.; Ghosh, A.; Avasthi, A.; Nehra, R.; Ahuja, C.K.; Khandelwal, N. Do neurocognitive functions in cannabis induced psychosis groups differ from schizophrenia with cannabis use? A controlled cross-sectional study. Int. J. Psychiatry Clin. Pract. 2021, 25, 283–291. [Google Scholar] [CrossRef]
- Benaiges, I.; Serra-Grabulosa, J.M.; Adan, A. Neuropsychological functioning and age-related changes in schizophrenia and/or cocaine dependence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bogaty, S.E.R.; Lee, R.S.C.; Hickie, I.B.; Hermens, D.F. Meta-analysis of neurocognition in young psychosis patients with current cannabis use. J. Psychiatr. Res. 2018, 99, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Cobia, D.J.; Wang, L.; Alpert, K.I.; Cronenwett, W.J.; Goldman, M.B.; Mamah, D.; Barch, D.M.; Breiter, H.C.; Csernansky, J.G. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophr. Bull. 2014, 40, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Palka, J.M.; Brown, E.S. Cognitive impairment in individuals with bipolar disorder with and without comorbid alcohol and/or cocaine use disorders. J. Affect. Disord. 2020, 272, 355–362. [Google Scholar] [CrossRef]
- Marshall, D.F.; Walker, S.J.; Ryan, K.A.; Kamali, M.; Saunders, E.F.H.; Weldon, A.L.; Adams, K.M.; McInnis, M.G.; Langenecker, S.A. Greater executive and visual memory dysfunction in comorbid bipolar disorder and substance use disorder. Psychiatry Res. 2012, 200, 252–257. [Google Scholar] [CrossRef][Green Version]
- Levy, B.; Manove, E.; Weiss, R.D. Recovery of cognitive functioning in patients with co-occurring bipolar disorder and alcohol dependence during early remission from an acute mood episode. Ann. Clin. Psychiatry 2012, 24, 143–154. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; Mellick, W.; Mitaro, E.; Tolliver, B.K. An evaluation of the impact of co-occurring anxiety and substance use disorder on bipolar disorder illness outcomes in STEP-BD. J. Affect. Disord. 2019, 246, 794–799. [Google Scholar] [CrossRef]
- Hermens, D.F.; Lee, R.S.C.; De Regt, T.; Lagopoulos, J.; Naismith, S.L.; Scott, E.M.; Hickie, I.B. Neuropsychological functioning is compromised in binge drinking young adults with depression. Psychiatry Res. 2013, 210, 256–262. [Google Scholar] [CrossRef]
- Liu, I.C.; Chiu, C.H.; Yang, T.T. The effects of gender and a co-occurring depressive disorder on neurocognitive functioning in patients with alcohol dependence. Alcohol Alcohol. 2010, 45, 231–236. [Google Scholar] [CrossRef]
- Uekermann, J.; Daum, I.; Schlebusch, P.; Wiebel, B.; Trenckmann, U. Depression and cognitive functioning in alcoholism. Addiction 2003, 98, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association DSM-5. Diagnostic and Statistical Manual of Mental Disorders; APA: Washington, DC, USA, 2013. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Entrevista Clínica Estructurada Para los Trastornos del Eje I del DSM-IV, Versión Clínica; Masson: Barcelona, Spain, 1999. [Google Scholar]
- Gálvez, B.P.; Fernández, L.G. Validación española del Drug Abuse Screening Test (DAST-20 y DAST-10). Health Addict. 2010, 10, 35–50. [Google Scholar]
- Peralta, V.; Cuesta, M.J. Validación de la escala de los síndromes positivo y negativo (PANSS) en una muestra de esquizofrénicos españoles. Actas Luso-Esp. Neurol. Psiquiatr. Cienc. Afines 1994, 22, 171–177. [Google Scholar]
- Colom, F.; Vieta, E.; Martínez-Arán, A.; García-García, M.; Reinares, M.; Torrent, C.; Goikolea, J.; Banús, S.; Salamero, M. Versión española de una escala de evaluación de la manía: Validez y fiabilidad de la escala de Young. Med. Clin. 2002, 119, 366–371. [Google Scholar] [CrossRef]
- Bobes, J.; Bulbena, A.; Luque, A.; Dal-Ré, R.; Ballesteros, J.; Ibarra, N. Evaluación psicométrica comparativa de las versiones en español de 6, 17 y 21 ítems de la Escala de valoración de Hamilton para la evaluación de la depresión. Med. Clin. 2003, 120, 693–700. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III: Test de Inteligencia Para Adultos de Wechsler; Paidós: Buenos Aires, Argentina, 2002. [Google Scholar]
- Boone, D.E. WAIS-R scatter with psychiatric inpatients: I Intrasubtest scatter. Psychol. Rep. 1992, 71, 483–487. [Google Scholar]
- Schmidt, L.M.; Hesse, M.; Lykke, J. The impact of substance use disorders on the course of schizophrenia—A 15-year follow-up study. Schizophr. Res. 2011, 130, 228–233. [Google Scholar] [CrossRef]
- Van der Elst, W.; van Boxtel, M.P.J.; van Breukelen, G.J.P.; Jolles, J. Rey’s verbal learning test: Normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J. Int. Neuropsychol. Soc. 2005, 11, 290–302. [Google Scholar] [CrossRef]
- Reed, J.C.; Reed, H.B.C. The Halstead—Reitan Neuropsychological Battery. In Contemporary Approaches to Neuropsychological Assessment. Critical Issues in Neuropsychology; Goldstein, G., Incagnoli, T.M., Eds.; Springer: Boston, MA, USA, 1997; pp. 93–129. [Google Scholar]
- Tamayo, F.; Casals-Coll, M.; Sánchez-Benavides, G.; Quintana, M.; Manero, R.M.; Rognoni, T.; Calvo, L.; Palomo, R.; Aranciva, F.; Peña-Casanova, J. Estudios normativos españoles en población adulta joven (Proyecto NEURONORMA jóvenes): Normas para las pruebas span verbal, span visuoespacial, letter-number sequencing, Trail Making Test y symbol digit modalities Test. Neurología 2012, 27, 319–329. [Google Scholar] [CrossRef]
- Heaton, R. WCST-CV4. Wisconsin Card Sorting Test®: Computer Version 4–Research Edition; Psychological Assessment Resources: Lutz, FL, USA, 2003. [Google Scholar]
- Humes, G.E.; Welsh, M.C.; Retzlaff, P.; Cookson, N. Towers of hanoi and london: Reliability and validity of two executive function tasks. Assessment 1997, 4, 249–257. [Google Scholar] [CrossRef]
- Benaiges, I.; Serra-Grabulosa, J.M.; Prat, G.; Adan, A. Executive functioning in individuals with schizophrenia and/or cocaine dependence. Hum. Psychopharmacol. 2013, 28, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hørlyck, L.D.; Obenhausen, K.; Jansari, A.; Ullum, H.; Miskowiak, K.W. Virtual reality assessment of daily life executive functions in mood disorders: Associations with neuropsychological and functional measures. J. Affect. Disord. 2021, 280, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Petersen, J.Z.; Ott, C.V.; Knorr, U.; Kessing, L.V.; Gallagher, P.; Robinson, L. Predictors of the discrepancy between objective and subjective cognition in bipolar disorder: A novel methodology. Acta Psychiatr. Scand. 2016, 134, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Serrano-Serrano, A.B.; Marquez-Arrico, J.E.; Navarro, J.F.; Martinez-Nicolas, A.; Adan, A. Circadian characteristics in patients under treatment for substance use disorders and severe mental illness (schizophrenia, major depression and bipolar disorder). J. Clin. Med. 2021, 10, 4388. [Google Scholar] [CrossRef]
- Marquez-Arrico, J.E.; Río-Martínez, L.; Navarro, J.F.; Prat, G.; Adan, A. Personality profile and clinical correlates of patients with substance use disorder with and without comorbid depression under treatment. Front. Psychiatry 2019, 10, 764. [Google Scholar] [CrossRef]
- Lynn Starr, H.; Bermak, J.; Mao, L.; Rodriguez, S.; Alphs, L. Comparison of long-acting and oral antipsychotic treatment effects in patients with schizophrenia, comorbid substance abuse, and a history of recent incarceration: An exploratory analysis of the PRIDE study. Schizophr. Res. 2018, 194, 39–46. [Google Scholar] [CrossRef]
- Abdel-Baki, A.; Ouellet-Plamondon, C.; Salvat, É.; Grar, K.; Potvin, S. Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Res. 2017, 247, 113–119. [Google Scholar] [CrossRef]
- Llanes-Álvarez, C.; Andrés-de Llano, J.M.; Álvarez-Navares, A.I.; Pastor-Hidalgo, M.T.; Roncero, C.; Franco-Martín, M.A. Trends in psychiatric hospitalization for alcohol and drugs in Castilla y León between 2005 and 2015. Adicciones 2022, 34, 189–196. [Google Scholar] [CrossRef]
- Sánchez-Niubò, A.; Sordo, L.; Barrio, G.; Indave, B.; Domingo-Salvany, A. Onset and progression of drug use in the general population of Catalonia, Spain. Adicciones 2020, 32, 32–39. [Google Scholar]
- European Monitoring Centre for Drug and Addiction (EMCDDA). European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-9497-742-7. [Google Scholar]
- Donati, F.L.; D’Agostino, A.; Ferrarelli, F. Neurocognitive and neurophysiological endophenotypes in schizophrenia: An overview. Biomark. Neuropsychiatry 2020, 3, 100017. [Google Scholar] [CrossRef]
- Helldin, L.; Kane, J.M.; Karilampi, U.; Norlander, T.; Archer, T. Remission and cognitive ability in a cohort of patients with schizophrenia. J. Psychiatr. Res. 2006, 40, 738–745. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.A.; Deldin, P.J.; Pester, B.; McInnis, M.G.; Langenecker, S.A.; Ryan, K.A. Cognitive flexibility: A trait of bipolar disorder that worsens with length of illness. J. Clin. Exp. Neuropsychol. 2017, 39, 979–987. [Google Scholar] [CrossRef]
- Hagen, E.; Erga, A.H.; Hagen, K.P.; Nesvåg, S.M.; McKay, J.R.; Lundervold, A.J.; Walderhaug, E. Assessment of executive function in patients with substance use disorder: A comparison of inventory and performance-based assessment. J. Subst. Abuse Treat. 2016, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Pérez-García, M. Ecological assessment of executive functions in substance dependent individuals. Drug Alcohol Depend. 2007, 90, 48–55. [Google Scholar] [CrossRef]
- Schulte, M.H.J.; Cousijn, J.; Den Uyl, T.E.; Goudriaan, A.E.; Van Den Brink, W.; Veltman, D.J.; Schilt, T.; Wiers, R.W. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin. Psychol. Rev. 2014, 34, 531–550. [Google Scholar] [CrossRef]
- Richard, Y.; Moustafa, A. Impulsive behavior in drug addiction: Clinical, cognitive, and neural correlates. In Cognitive, Clinical, and Neural Aspects of Drug Addiction; Moustafa, A.A., Ed.; Academic Press: London, UK, 2021; pp. 1–20. [Google Scholar]
- Prat, G.; Marquez-Arrico, J.E.; Río-Martínez, L.; Navarro, J.F.; Adan, A. Premorbid functioning in schizophrenia spectrum disorders with comorbid substance use: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 110, 110310. [Google Scholar] [CrossRef]
- Brewer, W.J.; Francey, S.M.; Wood, S.J.; Jackson, H.J.; Pantelis, C.; Phillips, L.J.; Young, A.R.; Anderson, V.A.; McGorry, P.P. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry 2005, 162, 71–78. [Google Scholar] [CrossRef]
- Okasha, T.A.; Hussein, H.; Shorub, E.; Nagi, H.; Moustafa, A.A.; El-Serafi, D. Cognitive dysfunction among inpatients and outpatients with schizophrenia: Relationship to positive and negative symptoms. Middle East Curr. Psychiatry 2020, 27, 58. [Google Scholar] [CrossRef]
- Fitapelli, B.; Lindenmayer, J.P. Advances in cognitive remediation training in schizophrenia: A review. Brain Sci. 2022, 12, 129. [Google Scholar] [CrossRef]
- Mogami, T. Cognitive remediation for schizophrenia with focus on NEAR. Front. Psychiatry 2018, 8, 304. [Google Scholar] [CrossRef] [PubMed]
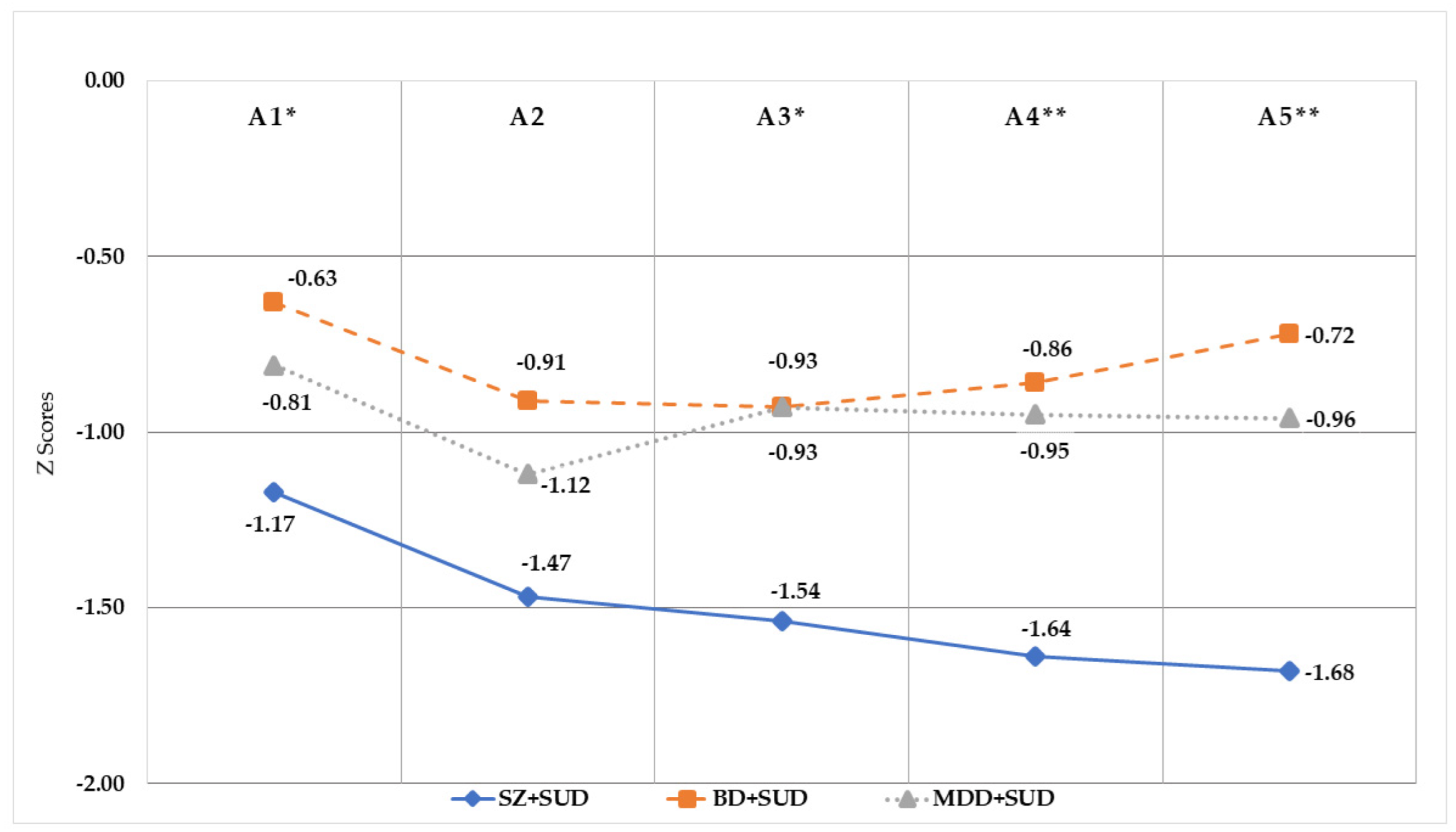

| Sociodemographic Data | SZ + SUD (N = 40) | BD + SUD (N = 40) | MDD + SUD (N = 40) | Contrasts |
|---|---|---|---|---|
| Age (years) | 34.93 ± 7.71 | 38.55 ± 8.59 | 39.80 ± 5.55 | F(2,119) = 4.68 * |
| Marital status | χ2(2) = 12.59 | |||
| Single | 82.5% | 55.0% | 50.0% | |
| Married/stable partner | 15.0% | 42.5% | 45.0% | |
| Separated/divorced | 2.5% | 2.5% | 5.0% | |
| Family situation | χ2(1) = 9.45 ** | |||
| Without children | 82.5% | 62.5% | 50.0% | |
| With children | 17.5% | 37.5% | 50.0% | |
| Living arrangements | χ2(3) = 21.87 *** | |||
| Alone | 7.5% | 17.5% | 5.0% | |
| Sharing | 67.5% | 62.5% | 47.5% | |
| Therapeutic community | 15.0% | 20.0% | 47.5% | |
| Supported accommodation | 10.0% | 0% | 0% | |
| Economic situation | χ2(3) = 35.37 *** | |||
| Working | 12.5% | 10.0% | 12.5% | |
| Unemployed | 20.0% | 20.0% | 57.5% | |
| Under sick leave | 7.5% | 5.0% | 17.5% | |
| Disability pension | 60.0% | 65.0% | 12.5% | |
| Years of schooling | 9.88 ± 2.43 | 11.25 ± 3.26 | 10.45 ± 2.05 | F(2,119) = 2.76 |
| Clinical Data | SZ + SUD (N = 40) | BD + SUD (N = 40) | MDD + SUD (N = 40) | Contrasts |
|---|---|---|---|---|
| SMI age of onset (years) | 23.88 ± 7.31 | 25.95 ± 8.82 | 31.83 ± 8.12 | F(2,119) = 10.34 *** |
| History of suicide attempts | 45.0% | 40.0% | 47.5% | χ2(1) = 0.47 |
| Pharmacological treatment a | ||||
| Quantity of medications per day | 3.36 ± 1.56 | 3.12 ± 1.77 | 2.33 ± 1.64 | F(2,119) = 4.13 * |
| Typical antipsychotic | 28.2% | 10.0% | 2.6% | χ2(1) = 15.19 * |
| Atypical antipsychotic | 94.6% | 65.0% | 22.5% | χ2(1) = 44.89 *** |
| Mood stabilizers | 41.0% | 67.5% | 32.4% | χ2(1) = 26.02 *** |
| Anxiolytics | 43.6% | 35.0% | 39.8% | χ2(1) = 2.14 |
| Antidepressants | 33.3% | 46.2% | 71.8% | χ2(1) = 15.48 * |
| Anticholinergic | 25.6% | 2.5% | 0% | χ2(1) = 18.25 *** |
| Alcohol-aversive-agent | 25.6% | 22.5% | 25.6% | χ2(1) = 0.14 |
| Other psychotropics | 13.2% | 12.5% | 17.9% | χ2(1) = 2.24 |
| Chlorpromazine equivalent dose (mg) | 422.30 ± 35.22 | 138.81 ± 3.27 | 43.15 ± 34.27 | F(2,119) = 31.79 *** |
| PANSS positive | 12.34 ± 6.20 | |||
| PANSS negative | 15.07 ± 7.54 | |||
| PANSS composite | −2.65 ± 5.83 | |||
| PANSS general psychopathology | 30.71 ± 11.62 | |||
| HDRS total score | 7.06 ± 5.17 | 11.10 ± 5.28 | F(1,79) = 12.39 *** | |
| YMRS total score | 3.16 ± 3.13 |
| Clinical Data | SZ + SUD (N = 40) | BD + SUD (N = 40) | MDD + SUD (N = 40) | Contrasts |
|---|---|---|---|---|
| SUD age of onset (years) | 17.50 ± 5.19 | 20.33 ± 7.51 | 18.55 ± 6.94 | F(2,119) = 3.39 |
| SUD duration (years) | 17.49 ± 7.57 | 18.23 ± 9.10 | 21.25 ± 8.59 | F(2,119) = 2.12 |
| Quantity of substances used | 3.74 ± 1.44 | 2.54 ± 1.19 | 2.95 ± 1.41 | F(2,119) = 7.55 *** |
| Polydrug use | 82.5% | 37.5% | 50.0% | χ2(1) = 38.67 * |
| Type of substance a | ||||
| Cocaine | 97.5% | 65.0% | 82.5% | χ2(1) = 14.14 *** |
| Alcohol | 75.0% | 87.5% | 90.0% | χ2(1) = 3.46 |
| Cannabis | 82.5% | 47.5% | 57.5% | χ2(1) = 11.09 ** |
| Ecstasy | 17.5% | 10.0% | 5.0% | χ2(1) = 3.27 |
| Hallucinogens | 40.0% | 15.0% | 20.0% | χ2(1) = 7.46 * |
| Opioids | 30.0% | 12.5% | 25.0% | χ2(1) = 3.72 |
| Anxiolytic/hypnotic-sedative | 32.5% | 10.0% | 12.5% | χ2(1) = 8.12 * |
| Daily cigarettes per day | 21.82 ± 11.29 | 19.35 ± 9.13 | 13.83 ± 7.36 | F(2,119) = 7.61 *** |
| Fagerström total score | 6.10 ± 2.60 | 5.03 ± 2.86 | 4.26 ± 2.41 | F(2,119) = 4.85 ** |
| DAST-20 total score | 12.89 ± 3.03 | 11.17 ± 4.98 | 13.47 ± 4.04 | F(2,119) = 2.35 |
| Severity of addiction | χ2(3) = 20.47 ** | |||
| Low | 3.7% | 20.8% | 3.3% | |
| Mild | 14.8% | 33.4% | 16.7% | |
| High | 70.4% | 25.0% | 50.0% | |
| Severe | 11.1% | 20.8% | 30.0% | |
| Abstinence period (months) | 11.13 ± 5.68 | 9.70 ± 6.69 | 8.22 ± 5.72 | F(2,119) = 0.74 |
| Quantity of relapses | 1.60 ± 3.38 | 0.90 ± 1.85 | 0.75 ± 1.30 | F(2,119) = 1.53 |
| SZ + SUD (N = 40) | BD + SUD (N = 40) | MDD + SUD (N = 40) | MANCOVA | ||
|---|---|---|---|---|---|
| F(2,119) | ηp2 | ||||
| RAVLT | |||||
| Total words | −2.00 ± 0.18 | −1.10 ± 0.19 | −1.11 ± 0.17 | 7.60 *** | 0.119 |
| Recog. list A/15 | −1.80 ± 0.24 | −0.98 ± 0.25 | −0.60 ± 0.23 | 6.25 ** | 0.097 |
| Trial Making Test | |||||
| TMT-A | −0.60 ± 0.19 | −0.58 ± 0.20 | −0.22 ± 0.18 | 1.58 | 0.027 |
| TMT-B | −0.70 ± 0.17 | −1.02 ± 0.18 | −0.27 ± 0.16 | 4.42 * | 0.073 |
| WSCT | |||||
| Categories completed | 5.53 ± 0.21 | 5.40 ± 0.19 | 5.43 ± 0.20 | 0.108 | 0.002 |
| Total errors | 19.73 ± 2.27 | 20.72 ± 2.25 | 20.55 ± 2.26 | 0.054 | 0.001 |
| Z scores | 0.76 ± 0.19 | 0.50 ± 0.18 | 0.45 ± 0.17 | 0.786 | 0.013 |
| Percentage of errors | 19.25 ± 1.55 | 19.10 ± 1.53 | 19.90 ± 1.54 | 0.076 | 0.001 |
| Z scores for percentage | 0.73 ± 0.20 | 0.62 ± 0.18 | 0.51 ± 0.17 | 0.359 | 0.006 |
| Perseverative errors | 5.46 ± 0.98 | 6.22 ± 0.97 | 6.67 ± 0.96 | 0.386 | 0.007 |
| Z scores | 2.19 ± 0.22 | 1.69 ± 0.21 | 1.59 ± 0.20 | 2.197 | 0.036 |
| Percentage perseverative errors | 4.71 ± 0.75 | 5.16 ± 0.74 | 5.90 ± 0.76 | 0.645 | 0.011 |
| Z scores for percentage | 2.45 ± 0.19 | 2.10 ± 0.20 | 1.95 ± 0.21 | 1.584 | 0.027 |
| Tower of Hanoi | |||||
| N° of movements | 25.46 ± 1.71 | 25.10 ± 1.78 | 29.05 ± 1.72 | 1.589 | 0.028 |
| Errors | 1.25 ± 0.39 | 1.49 ± 0.40 | 1.36 ± 0.39 | 0.910 | 0.002 |
| Response time | 211.35 ± 22.26 | 202.55 ± 23.27 | 246.42 ± 22.38 | 1.055 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez-Arrico, J.E.; Gonzalez-Sanchez, A.; Navarro, J.F.; Penadés, R.; Adan, A. Patients with Schizophrenia Showed Worse Cognitive Performance than Bipolar and Major Depressive Disorder in a Sample with Comorbid Substance Use Disorders. J. Clin. Med. 2022, 11, 6648. https://doi.org/10.3390/jcm11226648
Marquez-Arrico JE, Gonzalez-Sanchez A, Navarro JF, Penadés R, Adan A. Patients with Schizophrenia Showed Worse Cognitive Performance than Bipolar and Major Depressive Disorder in a Sample with Comorbid Substance Use Disorders. Journal of Clinical Medicine. 2022; 11(22):6648. https://doi.org/10.3390/jcm11226648
Chicago/Turabian StyleMarquez-Arrico, Julia E., Alvaro Gonzalez-Sanchez, José Francisco Navarro, Rafael Penadés, and Ana Adan. 2022. "Patients with Schizophrenia Showed Worse Cognitive Performance than Bipolar and Major Depressive Disorder in a Sample with Comorbid Substance Use Disorders" Journal of Clinical Medicine 11, no. 22: 6648. https://doi.org/10.3390/jcm11226648
APA StyleMarquez-Arrico, J. E., Gonzalez-Sanchez, A., Navarro, J. F., Penadés, R., & Adan, A. (2022). Patients with Schizophrenia Showed Worse Cognitive Performance than Bipolar and Major Depressive Disorder in a Sample with Comorbid Substance Use Disorders. Journal of Clinical Medicine, 11(22), 6648. https://doi.org/10.3390/jcm11226648






