Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
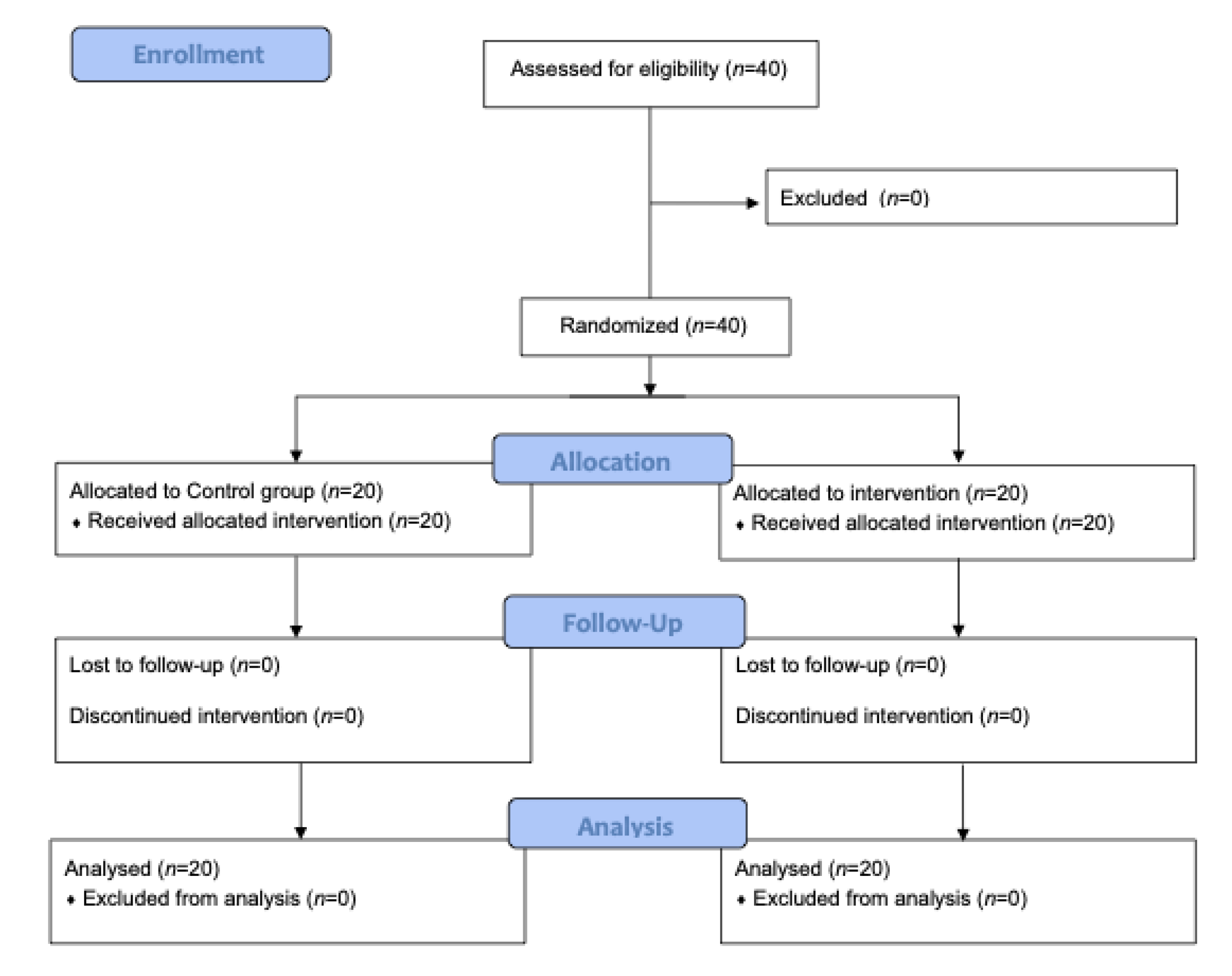
2.2. Randomization
2.3. Procedure
2.4. Outcomes
- -
- Number of hypotensive events;
- -
- Absolute time spent with hypotension during surgery (minutes);
- -
- Time spent in hypotension relative to surgical duration (%);
- -
- Time-weighted average of hypotension during surgery; measured by calculating the area under the threshold divided by the total duration of surgery. Practically, this parameter is calculated as the maximum depth of hypotension below the threshold of MAP < 65 mmHg (unit: mmHg) × total time spent in hypotension (unit: minutes) divided by total duration of surgery (unit: minutes). For example, a patient undergoing surgery lasting 100 min experiences 5 episodes of hypotension, all of them lasting 1 min, and all with a minimal MAP of 60 mmHg. In this case, the area under the threshold is 25 mmHg per minute (calculated as 5 min of hypotension × 5 mmHg of MAP < 65 mmHg). Finally, the time-weighted average will be 25 mmHg per minute divided by 100 min of surgery, corresponding to 0.25 mmHg.
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Hypotension-Related Outcomes
3.3. Biochemical Markers of Organ Injury and Oxidative Stress
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.T.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between Intraoperative Hypotension and Hypertension and 30-day Postoperative Mortality in Noncardiac Surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Wijeysundera, D.N.; Tait, G.A.; Beattie, W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015, 123, 515–523. [Google Scholar] [CrossRef]
- van Waes, J.A.; van Klei, W.A.; Wijeysundera, D.N.; van Wolfswinkel, L.; Lindsay, T.F.; Beattie, W.S. Association between Intraoperative Hypotension and Myocardial Injury after Vascular Surgery. Anesthesiology 2016, 124, 35–44. [Google Scholar] [CrossRef]
- Bijker, J.B.; van Klei, W.A.; Kappen, T.H.; van Wolfswinkel, L.; Moons, K.G.; Kalkman, C.J. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007, 107, 213–220. [Google Scholar] [CrossRef]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.; Villar, J.C.; Xavier, D.; Srinathan, S.; Guyatt, G.; Cruz, P.; Graham, M.; Wang, C.Y.; et al. Myocardial injury after noncardiac surgery: A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef]
- Landesberg, G.; Mosseri, M.; Shatz, V.; Akopnik, I.; Bocher, M.; Mayer, M.; Anner, H.; Berlatzky, Y.; Weissman, C. Cardiac troponin after major vascular surgery: The role of perioperative ischemia, preoperative thallium scanning, and coronary revascularization. J. Am. Coll Cardiol. 2004, 44, 569–575. [Google Scholar] [CrossRef][Green Version]
- Bax, J.J.; Baumgartner, H.; Ceconi, C.; Dean, V.; Deaton, C.; Fagard, R.; Funck-Brentano, C.; Hasdai, D.; Hoes, A.; Kirchhof, P. Third universal definition of myocardial infarction. J. Am. Coll Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef]
- Vernooij, L.M.; van Klei, W.A.; Machina, M.; Pasma, W.; Beattie, W.S.; Peelen, L.M. Different methods of modelling intraoperative hypotension and their association with postoperative complications in patients undergoing non-cardiac surgery. Br. J. Anaesth. 2018, 120, 1080–1089. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Bassetti, S.; Steuer, S.; Stelzig, C.; Hartwiger, S.; Biedert, S.; Schaub, N.; Buerge, C.; Potocki, M.; et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009, 361, 858–867. [Google Scholar] [CrossRef]
- Woods, D.R.; O’Hara, J.P.; Boos, C.J.; Hodkinson, P.D.; Tsakirides, C.; Hill, N.E.; Jose, D.; Hawkins, A.; Phillipson, K.; Hazlerigg, A.; et al. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur. J. Appl. Physiol. 2017, 117, 893–900. [Google Scholar] [CrossRef]
- Mir, I.N.; Chalak, L.F. Serum biomarkers to evaluate the integrity of the neurovascular unit. Early Hum. Dev. 2014, 90, 707–711. [Google Scholar] [CrossRef]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Woollard, J.R.; Herrmann, S.M.; Hickson, L.J.; Bendel, E.C.; Misra, S.; Glockner, J.; et al. Tissue hypoxia, inflammation, and loss of glomerular filtration rate in human atherosclerotic renovascular disease. Kidney Int. 2019, 95, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Wijnberge, M.; Geerts, B.F.; Hol, L.; Lemmers, N.; Mulder, M.P.; Berge, P.; Schenk, J.; Terwindt, L.E.; Hollmann, M.W.; Vlaar, A.P.; et al. Effect of a Machine Learning-Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery: The HYPE Randomized Clinical Trial. JAMA 2020, 323, 1052–1060. [Google Scholar] [CrossRef]
- Maheshwari, K.; Shimada, T.; Yang, D.; Khanna, S.; Cywinski, J.B.; Irefin, S.A.; Ayad, S.; Turan, A.; Ruetzler, K.; Qiu, Y.; et al. Hypotension Prediction Index for Prevention of Hypotension during Moderate- to High-risk Noncardiac Surgery. Anesthesiology 2020, 133, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Schneck, E.; Schulte, D.; Habig, L.; Ruhrmann, S.; Edinger, F.; Markmann, M.; Habicher, M.; Rickert, M.; Koch, C.; Sander, M. Hypotension Prediction Index based protocolized haemodynamic management reduces the incidence and duration of intraoperative hypotension in primary total hip arthroplasty: A single centre feasibility randomised blinded prospective interventional trial. J. Clin. Monit. Comput. 2020, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Vistisen, S.T.; Jian, Z.; Hatib, F.; Scheeren, T.W.L. Ability of an Arterial Waveform Analysis-Derived Hypotension Prediction Index to Predict Future Hypotensive Events in Surgical Patients. Anesth. Analg. 2020, 130, 352–359. [Google Scholar] [CrossRef]
- Wijnberge, M.; van der Ster, B.J.P.; Geerts, B.F.; de Beer, F.; Beurskens, C.; Emal, D.; Hollmann, M.W.; Vlaar, A.P.J.; Veelo, D.P. Clinical performance of a machine-learning algorithm to predict intra-operative hypotension with noninvasive arterial pressure waveforms: A cohort study. Eur. J. Anaesthesiol. 2021, 38, 609–615. [Google Scholar] [CrossRef]
- Shin, B.; Maler, S.A.; Reddy, K.; Fleming, N.W. Use of the Hypotension Prediction Index during Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1769–1775. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Berra, E.; Roux, D.; Richard, D.E.; Pouyssegur, J. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O(2)-driven proteasomal degradation irrespective of its subcellular localization: Nucleus or cytoplasm. EMBO Rep. 2001, 2, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Bialasiewicz, P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1alpha protein. J. Clin. Sleep Med. 2020, 16, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Jakkula, P.; Pettila, V.; Skrifvars, M.B.; Hastbacka, J.; Loisa, P.; Tiainen, M.; Wilkman, E.; Toppila, J.; Koskue, T.; Bendel, S.; et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 2018, 44, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Sieskiewicz, A.; Groblewska, M.; Lyson, T.; Piszczatowski, B.; Turek, G.; Rutkowski, R.; Mroczko, B.; Rogowski, M.; Mariak, Z. Neurobiochemical markers of brain ischemia in patients subjected to endoscopic skull base surgery under controlled hypotension. J. Neurosurg. Sci. 2016, 60, 313–319. [Google Scholar]
- Pfeifer, R.; Borner, A.; Krack, A.; Sigusch, H.H.; Surber, R.; Figulla, H.R. Outcome after cardiac arrest: Predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation 2005, 65, 49–55. [Google Scholar] [CrossRef]

| Intervention | Controls | p-Value | |
|---|---|---|---|
| Age (years, median) | 69.0 | 70.5 | 0.39 |
| BMI, (Kg/m2, median) | 25.3 | 25.6 | 0.15 |
| Gender, n (%) | |||
| Men | 10 (50%) | 12 (60%) | 0.62 |
| Women | 10 (50%) | 8 (40%) | |
| ASA classification, n (%) | |||
| I | 1 (5%) | 1 (5%) | 0.94 |
| II | 8 (40%) | 9 (45%) | |
| III | 11 (55%) | 10 (50%) | |
| Type of surgery, n (%) | |||
| Gastrointestinal | 18 (90%) | 16 (80%) | 0.81 |
| Gynecological | 1 (5%) | 2 (10%) | |
| Others * | 1 (5%) | 2 (10%) | |
| Comorbidities, n (%) | |||
| Hypertension | 10 (50%) | 14 (67%) | 0.22 |
| Type-2 diabetes | 2 (10%) | 4 (19%) | 0.35 |
| Others | 8 (40%) | 7 (33%) | 0.49 |
| Surgery duration, min (median) | 207.0 (64.0) | 237.0 (121.0) | 0.18 |
| Median (IQR) | Median Difference | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|
| Intervention | Controls | Lower | Higher | |||
| Number of hypotensive episodes, n | 3 (6) | 8 (13) | −5.0 | −9.0 | −0.5 | <0.001 |
| Total time spent in hypotension, min | 4.3 (11) | 21.3 (28) | −12.8 | −38.0 | −2.3 | <0.001 |
| Time in hypotension relative to surgical duration, % | 3.1 (6.4) | 7.8 (13.7) | −4.8 | −12.7 | 0.01 | 0.048 |
| Time-weighted average of hypotension, mmHg | 0.12 (0.35) | 0.37 (1.11) | −0.26 | −0.85 | −0.01 | 0.025 |
| NGAL | NSE | HIF-1α | S100B | Acetyl-CoA | hs Cardiac Troponin | LOOH | GSH | Hypotensive Episodies | |
|---|---|---|---|---|---|---|---|---|---|
| NGAL | 1.000 | - | - | - | - | - | - | - | - |
| NSE | 0.238 | 1.000 | - | - | - | - | - | - | - |
| HIF-1α | −0.284 | −0.369 * | 1.000 | - | - | - | - | - | - |
| S100B | 0.036 | 0.258 | −0.022 | 1.000 | - | - | - | - | - |
| Acetyl-CoA | −0.540 ** | −0.241 | −0.426 ** | 0.123 | 1.000 | - | - | - | - |
| Hs Cardiac Troponin | 0.356 * | 0.337 * | −0.304 | 0.030 | −0.391 * | 1.000 | - | - | - |
| LOOH | −0.136 | −0.103 | −0.40 | −0.258 | −0.010 | −0.040 | 1.000 | - | - |
| GSH | 0.041 | −0.213 | 0.253 | −0.283 | 0.114 | −0.398 ** | −0.099 | 1.000 | - |
| Hypotensive Episodies | 0.181 | 0.207 | −0.104 | 0.584 ** | −0.027 | 0.088 | −0.133 | −0.132 | 1.000 |
| Absolute time of hypotension | 0.288 | 0.159 | −0.133 | −0.628 ** | −0.111 | −0.138 | −0.121 | −0.164 | 0.883 ** |
| Relative time of hypotension | 0.278 | 0.118 | −0.148 | 0.612 ** | −0.096 | 0.127 | −0.199 | −0.179 | 0.880 ** |
| Time-weighted average of hypotension | 0.316 * | 0.093 | −0.150 | 0.575 ** | −0.153 | 0.174 | −0.160 | −0.170 | 0.820 ** |
| Intervention | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre Median (IQR) | Post Median (IQR) | Pre Median (IQR) | Post Median (IQR) | Median Difference | Lower 95% CI | Upper 95% CI | p-Value | |
| NGAL (ng/mL) | 2.1 (1.7) | 2.2 (1.8) | 2.2 (1.4) | 2.0 (1.0) | 0.24 | −0.27 | 0.84 | 0.528 |
| NSE (ng/mL) | 2.0 (1.3) | 1.0 (1.0) | 1.9 (1.9) | 1.7 (1.8) | −0.81 | −1.48 | −0.23 | 0.045 |
| HIF-1alpha (ng/mL) | 0.18 (0.12) | 0.15 (0.05) | 0.16 (0.11) | 0.15 (0.04) | −0.003 | −0.02 | 0.02 | 0.766 |
| S100B (pg/mL) | 0.36 (0.34) | 0.51 (0.63) | 0.32 (2.0) | 1.36 (1.29) | −0.76 | −1.21 | −0.42 | 0.622 |
| Acetyl–CoA (pmol/μL) | 0.11 (0.23) | 0.30 (1.4) | 0.16 (0.48) | 0.35 (2.0) | −0.09 | −0.84 | 0.36 | 0.509 |
| Human Cardiac Troponin 1 (pg/mL) | 41.8 (32.7) | 34.9 (36.9) | 32.1 (36.3) | 39.9 (39.2) | −4.58 | −31.2 | 4.7 | 0.584 |
| LOOH (nmol/μL) | 6.1 (7.6) | 4.4 (7.1) | 4.4 (5.0) | 3.9 (4.8) | −0.12 | −3.1 | 2.9 | 0.969 |
| GSH (nmol/μL) | 7.5 (7.0) | 7.0 (3.7) | 7.0 (5.5) | 5.0 (2.5) | 2.62 | 0.89 | 4.61 | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murabito, P.; Astuto, M.; Sanfilippo, F.; La Via, L.; Vasile, F.; Basile, F.; Cappellani, A.; Longhitano, L.; Distefano, A.; Li Volti, G. Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. J. Clin. Med. 2022, 11, 392. https://doi.org/10.3390/jcm11020392
Murabito P, Astuto M, Sanfilippo F, La Via L, Vasile F, Basile F, Cappellani A, Longhitano L, Distefano A, Li Volti G. Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(2):392. https://doi.org/10.3390/jcm11020392
Chicago/Turabian StyleMurabito, Paolo, Marinella Astuto, Filippo Sanfilippo, Luigi La Via, Francesco Vasile, Francesco Basile, Alessandro Cappellani, Lucia Longhitano, Alfio Distefano, and Giovanni Li Volti. 2022. "Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 2: 392. https://doi.org/10.3390/jcm11020392
APA StyleMurabito, P., Astuto, M., Sanfilippo, F., La Via, L., Vasile, F., Basile, F., Cappellani, A., Longhitano, L., Distefano, A., & Li Volti, G. (2022). Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine, 11(2), 392. https://doi.org/10.3390/jcm11020392









