A New Subform? Fast-Progressing, Severe Neurological Deterioration Caused by Spinal Epidural Lipomatosis
Abstract
1. Introduction
2. Materials and Methods
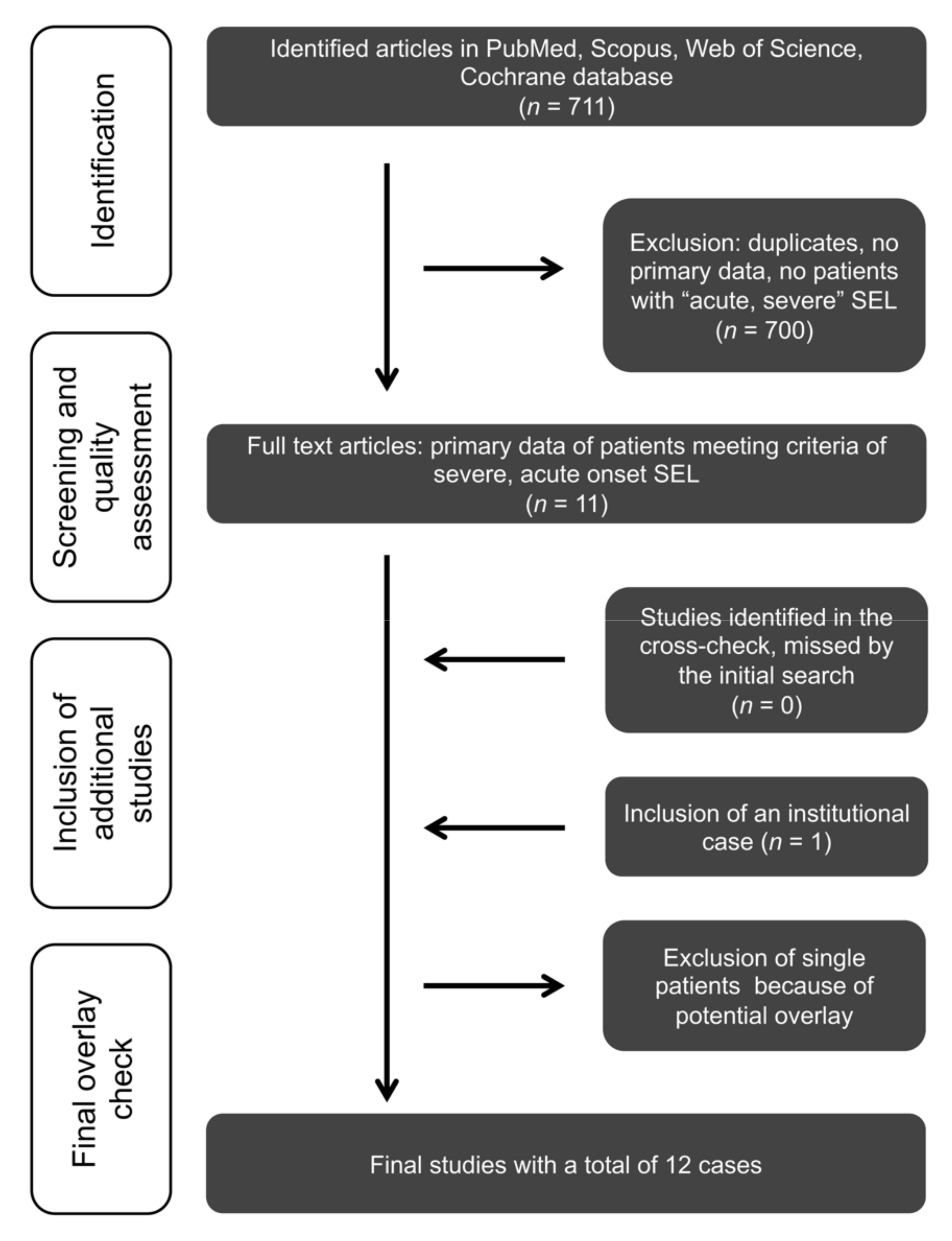
2.1. Systematic Review, Search Strategy, and Acquisition of the SEL Cohort Data
2.2. Data Analysis
2.2.1. Data Collection
2.2.2. Study Endpoints and Statistical Analysis
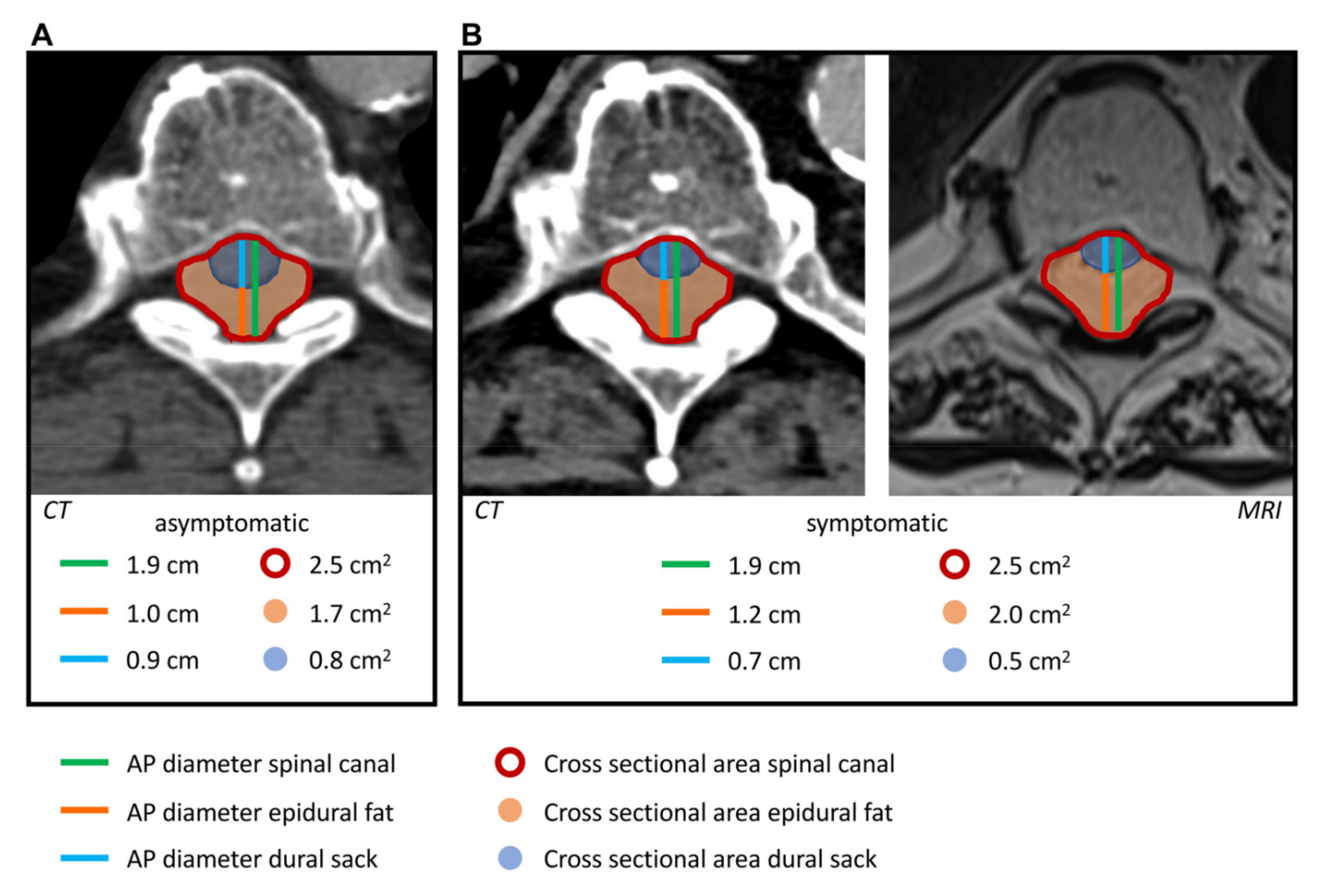
2.3. Image Analysis
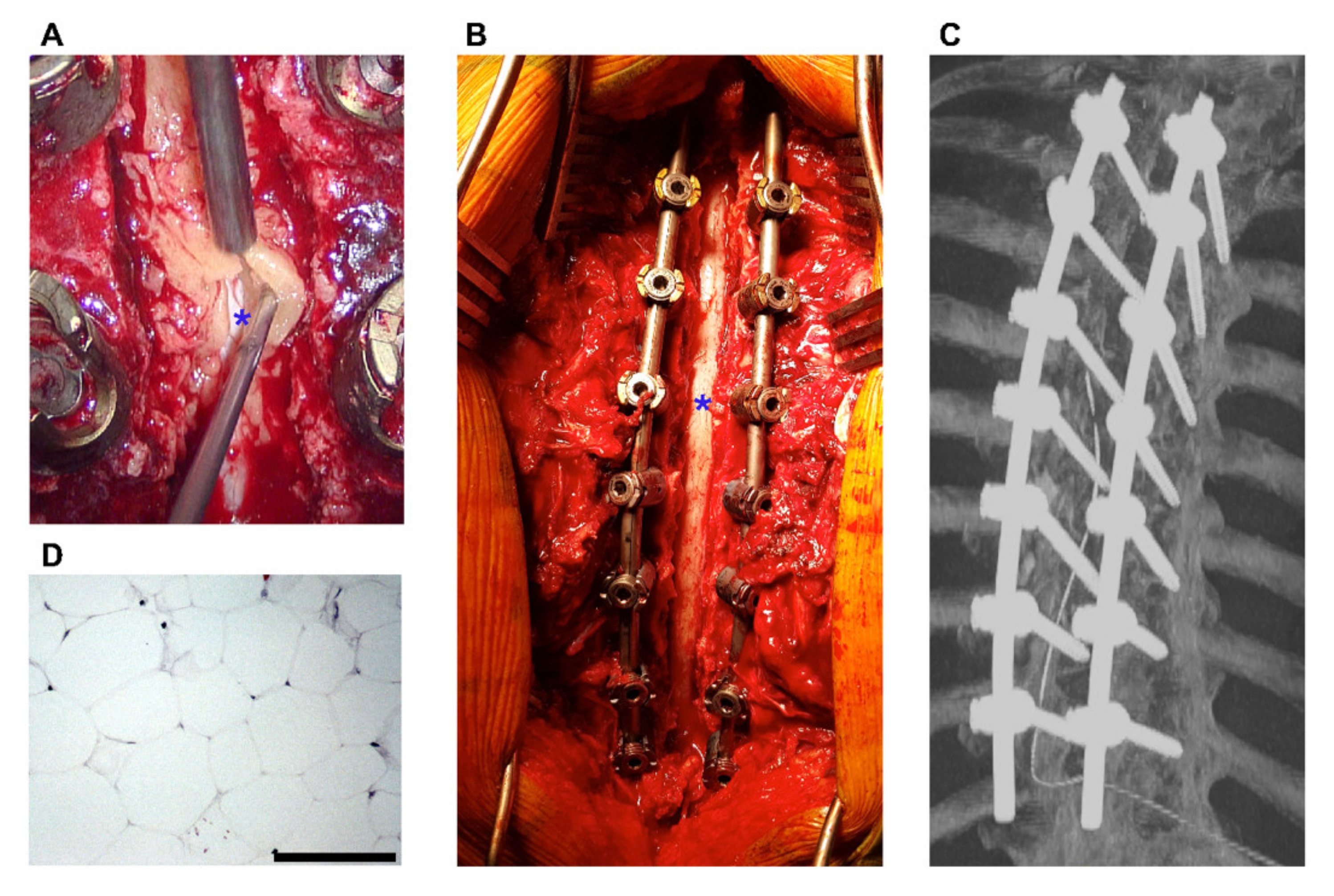
3. Results
Systematic Review of the Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.; Mendelis, J.; Cho, W. Spinal epidural lipomatosis: A review of pathogenesis, characteristics, clinical presentation, and management. Glob. Spine J. 2019, 6, 219256821879361. [Google Scholar] [CrossRef] [PubMed]
- Theyskens, N.C.; Pereira, N.R.P.; Janssen, S.J.; Bono, C.M.; Schwab, J.H.; Cha, T.D. The prevalence of spinal epidural lipomatosis on magnetic resonance imaging. Spine J. 2017, 17, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Boockvar, J.A.; Black, K.; Malik, S.; Stanek, A.; Tracey, K.J. Subacute paraparesis induced by venous thrombosis of a spinal angiolipoma. Spine 1997, 22, 2304–2308. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, I.S.; Song, Y.S.; Moon, J.I.; Song, J.W.; Kang, H. Dilatation of the spinal epidural venous plexus in patients with prominent epidural fat. Br. J. Radiol. 2016, 89, 20160064. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Fett, N.; Rosenbach, M.; Werth, V.P.; Micheletti, R.G. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: A review of glucocorticoid pharmacology and bone health. J. Am. Acad. Dermatol. 2017, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Resnick, I.B.; Gomori, J.M.; Kiselgoff, D.; Lossos, A.; Zilberman, I.; Miron, S.; Bitan, M.; Or, R.; Slavin, S.; Shapira, M.Y. Spinal epidural lipomatosis following haploidentical allogeneic bone marrow transplantation for non-hodgkin lymphoma. Clin. Transpl. 2004, 18, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.; Tibbles, C.; Friedberg, R. An unusual cause of spontaneous paralysis. J. Emerg. Med. 2009, 36, 290–295. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Jabbarli, R.; Dinger, T.F.; Pierscianek, D.; Oppong, M.D.; Chen, B.; Dammann, P.; Wrede, K.H.; Kaier, K.; Köhrmann, M.; Forsting, M.; et al. Intracranial aneurysms in sickle cell disease: A systematic review and case-control study. Curr. Neurovasc. Res. 2019, 16, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- McCormick, P.C.; Stein, B.M. Intramedullary tumors in adults. Neurosurg. Clin. N. Am. 1990, 1, 609–630. [Google Scholar] [CrossRef]
- Schneider, C.; Hidalgo, E.T.; Schmitt-Mechelke, T.; Kothbauer, K.F. Quality of life after surgical treatment of primary intramedullary spinal cord tumors in children. J. Neurosurg. Pediatrics 2014, 13, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Borré, D.G.; Borré, G.E.; Aude, F.; Palmieri, G.N. Lumbosacral epidural lipomatosis: MRI grading. Eur. Radiol. 2003, 13, 1709–1721. [Google Scholar] [CrossRef]
- Toshniwal, P.K.; Glick, R.P. Spinal epidural lipomatosis: Report of a case secondary to hypothyroidism and review of literature. J. Neurol. 1987, 234, 172–176. [Google Scholar] [CrossRef]
- Buthiau, D.; Piette, J.C.; Ducerveau, M.N.; Robert, G.; Godeau, P.; Heitz, F. Steroid-induced spinal epidural Lipomatosis: CT survey. J. Comput. Assist. Tomo. 1988, 12, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.G.; Barasch, E.; Hirschfeld, A.; Ross, L.; Einberg, K.; Gordon, M. Spinal epidural lipomatosis: A serious complication of iatrogenic cushing’s syndrome. Neurology 1989, 39, 1031. [Google Scholar] [CrossRef] [PubMed]
- Meisheri, Y.V.; Mehta, S.; Chattopadhyay, K. Acute paraplegia due to an extradural spinal lipoma: Case report. Spinal Cord 1996, 34, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Vince, G.H.; Brucker, C.; Langmann, P.; Herbold, C.; Solymosi, L.; Roosen, K. Epidural spinal lipomatosis with acute onset of paraplegia in an HIV-positive patient treated with corticosteroids and protease inhibitor: Case report. Spine 2005, 30, E524–E527. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, A.; Birbilis, T.; Gymnopoulou, E.; Prassopoulos, P. Paget disease of the spine manifested by thoracic and lumbar epidural lipomatosis. Spine 2007, 32, E789–E792. [Google Scholar] [CrossRef]
- López-González, A.; Giner, M.R. Idiopathic spinal epidural lipomatosis: Urgent decompression in an atypical case. Eur. Spine J. 2008, 17, 225–227. [Google Scholar] [CrossRef][Green Version]
- Stephenson, W.; Kauflin, M. Unusual presentation of spinal lipomatosis. Int. Med. Case Rep. J. 2014, 7, 139–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardivo, V.; Scudieri, C.; Bruzzo, M.; Lupidi, F. Acute neurologic decline in a patient with spinal stenosis: Blame it on the epidural fat. Brit. J. Neurosurg. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.A.; Doppman, J.L.; Patronas, N.J.; Nieman, L.K.; Chrousos, G.P. Do glucocorticoids cause spinal epidural lipomatosis? When endocrinology and spinal surgery meet. Trends Endocrinol. Metab. 2000, 11, 86–90. [Google Scholar] [CrossRef]
- Haid, R.W.; Kaufman, H.H.; Schochet, S.S.; Marano, G.D. Epidural lipomatosis simulating an epidural abscess: Case report and literature review. Neurosurgery 1987, 21, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Fessler, R.G.; Johnson, D.L.; Brown, F.D.; Erickson, R.K.; Reid, S.A.; Kranzler, L. Epidural lipomatosis in steroid-treated patients. Spine 1992, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, K.; Sheinart, K.; Lidov, M.; Cohen, B. Symptomatic spinal epidural lipomatosis in a patient with Cushing’s disease. Neurology 1995, 45, 2281–2283. [Google Scholar] [CrossRef]
- Akhaddar, A.; Ennouali, H.; Gazzaz, M.; Naama, O.; Elmostarchid, B.; Boucetta, M. Idiopathic spinal epidural lipomatosis without obesity: A case with relapsing and remitting course. Spinal Cord 2007, 46, 243–244. [Google Scholar] [CrossRef]
- Gala, F.B.; Aswani, Y. Imaging in spinal posterior epidural space lesions: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 299. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, A.G.L.; Groeneveld, W.; van der Linden, A.N.; Haak, H.R. Symptomatic epidural lipomatosis in ectopic Cushing’s syndrome. Eur. J. Endocrinol. 2004, 151, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.W.; Soane, T.; Gibson, R.; Pal, S.; Lees, C.W. Bilateral lower limb weakness in acute severe ulcerative colitis. Lancet 2016, 388, 101–102. [Google Scholar] [CrossRef]
- Lakomkin, N.; Zuckerman, S.L.; Stannard, B.; Montejo, J.; Sussman, E.S.; Virojanapa, J.; Kuzmik, G.; Goz, V.; Hadjipanayis, C.G.; Cheng, J.S. Preoperative risk stratification in spine tumor surgery: A comparison of the modified charlson index, frailty index, and ASA score. Spine 2019, 44, E782–E787. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, R.G.; Stephen, J.H.; Vernick, C.; Campbell, P.G.; Yadla, S.; Ghobrial, G.M.; Maltenfort, M.G.; Ratliff, J.K. ASA grade and Charlson comorbidity index of spinal surgery patients: Correlation with complications and societal costs. Spine J. 2013, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]

| ID | Sex | Age | Medical History | Steroids | mCCI | mMcCS | Spinal Levels | Surgery | Histo | Complications | Time [h] till | Ref. | Year | QAS * | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADM | f/u | Severe | Diagnosis | Treatment | |||||||||||||
| 1 | M | 45 | Hypothyroidism, obesity. | X | 0 | IV | IV | 11 | √ | √ | Death | 48 | 48 | N. r. | Toshniwal et al. [14] | 1987 | 28 |
| 2 | F | 62 | CAD, phlebitis, Raynaud’s syndrome, dermatomyositis. | √ | 0 | V | I | 5 | √ | X | No | “Rapidly progressive” | “Rapidly progressive” | N. r. | Buthiau et al. [15] | 1988 | 28 |
| 3 | M | 52 | Atopic dermatitis, CS with old fracture of T7. | √ | 0 | V | - | 4 | √ | X | Death | 0 | 12 | 23 | Kaplan et al. [16] | 1989 | 28 |
| 4 | M | 20 | None. | X | 0 | V | V | 4 | √ | √ | No | 12 | 96 | 120 | Meisheri et al. [17] | 1996 | 28 |
| 5 | M | 27 | NHL, BMT, GvHD, pneumonia, CS, obesity. | √ | 2 | IV | - | 19 | X | X | Death | “few days” | “few days” | N.t. | Resnick et al. [6] | 2004 | 24 |
| 6 | M | 41 | HIV, metastasized NSCLC. | √ | 10 | III | II | 9 | √ | X | No | 72 | 72 | 84 | Vince et al. [18] | 2005 | 30 |
| 7 | M | 60 | CAD, Paget’s disease. | X | 2 | IV | I | 6 | √ | √ | No | 72 | 72 | 72 | Oikonomou et al. [19] | 2007 | 30 |
| 8 | M | 55 | AHT. | X | 0 | IV | II | 5 | √ | √ | No | 24 | 48 | 48 | López-González et al. [20] | 2008 | 30 |
| 9 | M | 49 | DM I, hepatitis C, i.v. heroin abuse, tobacco (30py). | X | 2 | N.r. | N.r. | 13 | √ | X | No | 12 | 12 | 12 | Birmingham et al. [7] | 2009 | 26 |
| 10 | F | 35 | DM I, renal disease, endocarditis. | X | 4 | IV | II | 9 | √ | √ | No | “wake up” | 12 | 12 | Stephenson et al. [21] | 2014 | 28 |
| 11 | M | 69 | Obesity, COPD, DM II, AHT, hypercholesterolemia. | X | 4 | II | ≤ II | 2 | √ | √ | No | “acute” | N.r. | N.r. | Tardivo et al. [22] | 2021 | 26 |
| 12 | M | 67 | Adiposity, DM II, alcohol abuse, tobacco (40py), NSCLC. | √ | 8 | IV | III | 8 | √ | √ | Wound dehiscence, death | 24 | 72 | 72 | Suppl. Materials | 2022 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinger, T.F.; Eerikäinen, M.S.; Michel, A.; Gembruch, O.; Darkwah Oppong, M.; Chihi, M.; Blau, T.; Uerschels, A.-K.; Pierscianek, D.; Deuschl, C.; et al. A New Subform? Fast-Progressing, Severe Neurological Deterioration Caused by Spinal Epidural Lipomatosis. J. Clin. Med. 2022, 11, 366. https://doi.org/10.3390/jcm11020366
Dinger TF, Eerikäinen MS, Michel A, Gembruch O, Darkwah Oppong M, Chihi M, Blau T, Uerschels A-K, Pierscianek D, Deuschl C, et al. A New Subform? Fast-Progressing, Severe Neurological Deterioration Caused by Spinal Epidural Lipomatosis. Journal of Clinical Medicine. 2022; 11(2):366. https://doi.org/10.3390/jcm11020366
Chicago/Turabian StyleDinger, Thiemo Florin, Maija Susanna Eerikäinen, Anna Michel, Oliver Gembruch, Marvin Darkwah Oppong, Mehdi Chihi, Tobias Blau, Anne-Kathrin Uerschels, Daniela Pierscianek, Cornelius Deuschl, and et al. 2022. "A New Subform? Fast-Progressing, Severe Neurological Deterioration Caused by Spinal Epidural Lipomatosis" Journal of Clinical Medicine 11, no. 2: 366. https://doi.org/10.3390/jcm11020366
APA StyleDinger, T. F., Eerikäinen, M. S., Michel, A., Gembruch, O., Darkwah Oppong, M., Chihi, M., Blau, T., Uerschels, A.-K., Pierscianek, D., Deuschl, C., Jabbarli, R., Sure, U., & Wrede, K. H. (2022). A New Subform? Fast-Progressing, Severe Neurological Deterioration Caused by Spinal Epidural Lipomatosis. Journal of Clinical Medicine, 11(2), 366. https://doi.org/10.3390/jcm11020366








