Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Study Selection and Quality Assessment
3.2. Study Characteristics
3.3. Primary Endpoints: Overall Survival (OS), Cancer-Specific Survival (CSS), and Disease-Free Survival (DFS)
3.4. Primary Endpoints: Recurrence Patterns
3.5. Secondary Endpoints: Perioperative Morbidities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020. 2020. Available online: https://www.iarc.fr/fr/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/ (accessed on 1 May 2021).
- National Comprehensive Cancer Network. NCCN Guidelines—Non–Small Cell Lung Cancer, Version 4.2021. 2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 1 May 2021).
- Wang, W.; Sun, Y.; Li, H.; Bao, M.; Liu, X.; Jiang, G.; Ye, C.; Hu, Y. Surgical modality for stage IA non-small cell lung cancer among the elderly: Analysis of the Surveillance, Epidemiology, and End Results database. J. Thorac. Dis. 2020, 12, 6731–6742. [Google Scholar] [CrossRef]
- Shirvani, S.M.; Jiang, J.; Chang, J.Y.; Welsh, J.; Likhacheva, A.; Buchholz, T.A.; Swisher, S.G.; Smith, B.D. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014, 149, 1244–1253. [Google Scholar] [CrossRef]
- Stokes, W.A.; Bronsert, M.R.; Meguid, R.A.; Blum, M.G.; Jones, B.L.; Koshy, M.; Sher, D.J.; Louie, A.V.; Palma, D.A.; Senan, S.; et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 642–651. [Google Scholar] [CrossRef]
- Keenan, R.J.; Landreneau, R.J.; Maley, R.H., Jr.; Singh, D.; Macherey, R.; Bartley, S.; Santucci, T. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann. Thorac. Surg. 2004, 78, 228–233; discussion 228–233. [Google Scholar] [CrossRef]
- Ijsseldijk, M.A.; Shoni, M.; Siegert, C.; Seegers, J.; van Engelenburg, A.K.; Tsai, T.C.; Lebenthal, A.; Ten Broek, R.P. Oncological Outcomes of Lobar Resection, Segmentectomy, and Wedge Resection for T1a Non-Small-Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 582–590. [Google Scholar] [CrossRef]
- Winckelmans, T.; Decaluwe, H.; De Leyn, P.; Van Raemdonck, D. Segmentectomy or lobectomy for early-stage non-small-cell lung cancer: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2020, 57, 1051–1060. [Google Scholar] [CrossRef]
- Chan, E.G.; Chan, P.G.; Mazur, S.N.; Normolle, D.P.; Luketich, J.D.; Landreneau, R.J.; Schuchert, M.J. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2021, 161, 1639–1648.e2. [Google Scholar] [CrossRef]
- Hao, B.; Zhang, L.; Fan, T.; Liu, B.; Jiang, W.; Hu, H.; Geng, Q. Survival Following Segmentectomy or Lobectomy in Patients With Stage IB Non-small-cell Lung Cancer. Front. Oncol. 2020, 10, 661. [Google Scholar] [CrossRef]
- Onaitis, M.W.; Furnary, A.P.; Kosinski, A.S.; Kim, S.; Boffa, D.; Tong, B.C.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Putnam, J.B., Jr.; et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann. Thorac. Surg. 2018, 105, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Mimae, T.; Miyata, Y.; Tsutani, Y.; Imai, K.; Ito, H.; Nakayama, H.; Ikeda, N.; Okada, M. Wedge resection as an alternative treatment for octogenarian and older patients with early-stage non-small-cell lung cancer. Jpn. J. Clin. Oncol. 2020, 50, 1051–1057. [Google Scholar] [CrossRef]
- Chen, T.; Luo, J.; Wang, R.; Gu, H.; Gu, Y.; Huang, Q.; Wang, Y.; Zheng, J.; Yang, Y.; Zhao, H. Prognosis of limited resection versus lobectomy in elderly patients with invasive lung adenocarcinoma with tumor size less than or equal to 2 cm. J. Thorac. Dis. 2018, 10, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Tsutani, Y.; Tsubokawa, N.; Ito, M.; Misumi, K.; Hanaki, H.; Miyata, Y.; Okada, M. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2018, 53, 366–371. [Google Scholar] [CrossRef]
- Hou, B.; Deng, X.F.; Zhou, D.; Liu, Q.X.; Dai, J.G. Segmentectomy versus wedge resection for the treatment of high-risk operable patients with stage I non-small cell lung cancer: A meta-analysis. Ther. Adv. Respir. Dis. 2016, 10, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.; Landreneau, R.; Mandrekar, S.; Hillman, S.; Nichols, F.; Jones, D.; Starnes, S.; Tan, A.; Putnam, J.; Meyers, B.; et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann. Thorac. Surg. 2013, 96, 1747–1754; discussion 1754–1755. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sui, X.; Chen, X.; Zhang, L.; Wang, X.; Wang, S.; Wang, J. Sublobar resection versus lobectomy in Surgical Treatment of Elderly Patients with early-stage non-small cell lung cancer (STEPS): Study protocol for a randomized controlled trial. Trials 2016, 17, 191. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (accessed on 1 May 2021).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Williamson, P.R.; Smith, C.T.; Hutton, J.L.; Marson, A.G. Aggregate data meta-analysis with time-to-event outcomes. Stat. Med. 2002, 21, 3337–3351. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Vazirani, J.; Moraes, J.; Barnett, S.; Johnson, D.F.; Knight, S.; Miller, A.; Wright, G.; Alam, N.Z.; Conron, M.; Irving, L.B.; et al. Outcomes following resection of non-small cell lung cancer in octogenarians. ANZ J. Surg. 2018, 88, 1322–1327. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, G.; Xu, J.; Cui, L.; Dong, W.; Ni, Y.; Qu, X.; Du, J. Sublobectomy versus lobectomy for stage I non-small cell lung cancer in the elderly. Int. J. Surg. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Fiorelli, A.; Caronia, F.P.; Daddi, N.; Loizzi, D.; Ampollini, L.; Ardò, N.; Ventura, L.; Carbognani, P.; Potenza, R.; Ardissone, F.; et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: An appropriate choice in elderly patients? Surg. Today 2016, 46, 1370–1382. [Google Scholar] [CrossRef]
- Fang, Z.; He, J.; Fang, W.; Ruan, L.; Fang, F. Long-term Outcomes of Thoracoscopic Anatomic Resections and Systematic Lymphadenectomy for Elderly High-risk Patients with Stage IB Non-small-cell Lung Cancer. Heart Lung Circ. 2016, 25, 392–397. [Google Scholar] [CrossRef]
- Dell’Amore, A.; Monteverde, M.; Martucci, N.; Sanna, S.; Caroli, G.; Dolci, G.; Dell’Amore, D.; Rocco, G. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: Factors affecting surgical outcomes and long-term results. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, H.; Li, Y. Early lung cancer in the elderly: Sublobar resection provides equivalent long-term survival in comparison with lobectomy. Contemp. Oncol. 2014, 18, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Hu, D.; Zhong, C.; Zhao, H. Safety and efficacy of thoracoscopic wedge resection for elderly high-risk patients with stage I peripheral non-small-cell lung cancer. J. Cardiothorac. Surg. 2013, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Warwick, R.; Mediratta, N.; Shackcloth, M.; Page, R.; McShane, J.; Shaw, M.; Poullis, M. Wedge resection verses lobectomy for stage 1 non-small-cell lung cancer. Asian Cardiovasc. Thorac. Ann. 2013, 21, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Hirono, T.; Watanabe, T. Safety and prognosis of limited surgery for octogenarians with non-small-cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Schuchert, M.J.; Awais, O.; Abbas, G.; Horne, Z.D.; Nason, K.S.; Pennathur, A.; Souza, A.P.; Siegfried, J.M.; Wilson, D.O.; Luketich, J.D.; et al. Influence of age and IB status after resection of node-negative non-small cell lung cancer. Ann. Thorac. Surg. 2012, 93, 929–935; discussion 935–936. [Google Scholar] [CrossRef]
- Okami, J.; Ito, Y.; Higashiyama, M.; Nakayama, T.; Tokunaga, T.; Maeda, J.; Kodama, K. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann. Thorac. Surg. 2010, 90, 1651–1656. [Google Scholar] [CrossRef]
- Kilic, A.; Schuchert, M.J.; Pettiford, B.L.; Pennathur, A.; Landreneau, J.R.; Landreneau, J.P.; Luketich, J.D.; Landreneau, R.J. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann. Thorac. Surg. 2009, 87, 1662–1666; discussion 1667–1668. [Google Scholar] [CrossRef]
- Ghosh, S. Long term results of surgery versus continuous hyperfractionated accelerated radiotherapy (CHART) in patients aged >70 years with stage 1 non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2003, 24, 1002–1007. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, P.; Wang, Y.; Xu, J.; Yuan, X.; Wang, Z.; Lv, W.; Hu, J. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 105, 1483–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onaitis, M.W.; Furnary, A.P.; Kosinski, A.S.; Feng, L.; Boffa, D.; Tong, B.C.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Habib, R.; et al. Equivalent Survival Between Lobectomy and Segmentectomy for Clinical Stage IA Lung Cancer. Ann. Thorac. Surg. 2020, 110, 1882–1891. [Google Scholar] [CrossRef]
- Cao, C.; Wang, D.; Chung, C.; Tian, D.; Rimner, A.; Huang, J.; Jones, D.R. A systematic review and meta-analysis of stereotactic body radiation therapy versus surgery for patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 157, 362–373.e8. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, W.; Zhang, J.; You, G.; Xu, J.; Yu, D.; Peng, J.; Wei, Y. Systematic review and meta-analysis of video-assisted thoracoscopic surgery segmentectomy versus lobectomy for stage I non-small cell lung cancer. World J. Surg. Oncol. 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, G.; Leschber, G.; Schwarz, B.; Brintrup, D.L.; Ose, C.; Weinreich, G.; Passlick, B.; Hecker, E.; Kugler, C.; Dienemann, H.; et al. Perioperative course and quality of life in a prospective randomized multicenter phase III trial, comparing standard lobectomy versus anatomical segmentectomy in patients with non-small cell lung cancer up to 2cm, stage IA (7th edition of TNM staging system). Lung Cancer 2019, 138, 19–26. [Google Scholar] [CrossRef]
- Castaneda, C.; Charnley, J.M.; Evans, W.J.; Crim, M.C. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am. J. Clin. Nutr. 1995, 62, 30–39. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Kamigaichi, A.; Tsutani, Y.; Kagimoto, A.; Fujiwara, M.; Mimae, T.; Miyata, Y.; Okada, M. Comparing Segmentectomy and Lobectomy for Clinical Stage IA Solid-dominant Lung Cancer Measuring 2.1 to 3 cm. Clin. Lung Cancer 2020, 21, e528–e538. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.I.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef]



| Author Year | Database/Country | Study Design | Study Period | Female (%) | Age Cutoff | Cancer Stage (TNM) | Surgical Resections | Systematic Lymph Node Dissection/Sampling | Reasons for Sub | Patient Characteristics † | Disease Characteristics † | Extracted Endpoints | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lob | Sub | Seg | Wed | Age | FEV1 | DLCO | COPD | OCM | Size | p Stage | Histology | LNR | ||||||||||
| Database studies | ||||||||||||||||||||||
| Wang et al. 2020 | SEER | Retro | 1998–2016 | 55.1 | 70- | c I ≤ 3 cm (TNM v.8) | 3279 | 2918 | 620 | 2298 | NR | NR | Y | Y | Y | N | OS, CSS | |||||
| Onaitis et al. 2018 | STS-GTSD | Retro | 2002–2013 | NR | 65- | c I (NR) | A total of 20,635 | NR | NR | OS | ||||||||||||
| Stokes et al. 2018 | NCDB | Retro | 2004–2013 | 55.1 | 75- | c I ≤ 5 cm (TNM v.7) | 11,993 | 4537 | NR | NR | NR | NR | Mortality | |||||||||
| Shirvani et al. 2015 | SEER | Retro | 2003–2009 | 53.4 | 66- | p I ≤ 5 cm (TNM v.7) | 7215 | 1496 | NR | NR | NR | NR | Y | Y | Y | Y | Y | N | Y | Mortality | ||
| Cohort studies | ||||||||||||||||||||||
| Mimae et al. 2020 | Japan | Retro PM | 2010–2016 | 46.6 | 80- | c I ≤ 2 cm (TNM v.8) | 21 | 37 | 9 | 28 | Lob/Seg: Yes Wed: No | I, C | N | N | N | N | N | N | Y | OS, DFS | ||
| Chen et al. 2018 ‡ | China | Retro | 2009–2015 | 56.5 | 65- | p I ≤ 2 cm (TNM v.8) | 442 | 224 | 58 | 166 | Lob/Seg: Yes Wed: No | I, C | Y | N | N | OS, DFS | ||||||
| Tsutani et al. 2018 | Japan | Retro PM | 2007–2015 | 42.4 | 75- | c I ≤ 5 cm (TNM v.7) | 106 | 99 | 56 | 43 | NR | I, C | N | N | Y | N | Y | Y | N | OS, DFS, recurrence, complications | ||
| Vazirani et al. 2018 | Australia | Retro | 2005–2016 | NR | 80- | c I ≤ 5 cm (TNM v.7) | 121 | 79 | 34 | 45 | Lob/Seg: Yes Wed: No | NR | N | N | OS, recurrence, mortality | |||||||
| Qiu et al. 2017 | China | Retro | 2006–2012 | 29.8 | 65- | p I ≤ 5 cm (TNM v.7) | 206 | 39 | NR | NR | Lob/Seg: Yes Wed: No | I, C | N | Y | Y | N | Y | Y | Y | OS, DFS, mortality, complications | ||
| Fiorelli et al. 2016 | Italy | Retro PM | 2006–2012 | 38.5 | 75- | c I ≤ 5 cm (TNM v.7) | 149 | 90 | 39 | 51 | Lob/Seg: Yes Wed: No | C | N | Y | Y | Y | Y | N | Y | N | Y | OS, CSS, DFS, recurrence, mortality, complications |
| Dell’Amore et al. 2015 | Italy | Retro | 2000–2010 | 20.5 | 80- | c I ≤ 3 cm (TNM v.7) | 29 | 27 | NR | NR | Y | Y | Y | N | N | N | OS, mortality, complications | |||||
| Fang et al. 2015 | China | Retro | 2008–2010 | 52.1 | 65- | p IB 3–5 cm (TNM v.7) | 126 | - | 116 | - | Lob/Seg: Yes | C | N | N | N | N | OS, DFS, recurrence, complications | |||||
| Liu et al. 2014 | China | Retro | 2004–2010 | 42.5 | 70- | p I ≤ 5 cm (TNM v.7) | 122 | 45 | NR | NR | NR | C | OS | |||||||||
| Lin et al. 2013 ‡ | China | Retro | 2008–2012 | 38.3 | 70- | p I ≤ 5 cm (TNM v.7) | 33 | - | - | 14 | Lob/Seg: Yes Wed: No | C | N | N | N | N | Recurrence, mortality, complications | |||||
| Warwick et al. 2013 | UK | Retro PM | 2001–2011 | 50.5 | 70- | p I ≤ 5 cm (TNM v.7) | 152 | - | - | 83 | Lob: Yes Wed: No | C | Y | Y | Y | Y | N | N | OS, mortality | |||
| Okada et al. 2012 | Japan | Retro | 1996–2008 | 31.8 | 80- | c I ≤ 7 cm (TNM v.6) | 14 | 20 | 7 | 13 | Lob/Seg: Yes Wed: No | C | OS, CSS | |||||||||
| Schuchert et al. 2012 ‡ | USA | Retro | 1999–2010 | 51.4 | 70- | p I ≤ 7 cm (TNM v.6) | 290 | - | 171 | - | Lob/Seg: Yes | I, C | N | Y | Y | N | Y | N | Y | Recurrence, mortality, complications | ||
| Okami et al. 2010 | Japan | Retro | 1991–2007 | 35.8 | 75- | p I ≤ 3 cm (TNM v.7) | 82 | 54 | 33 | 21 | Lob/Seg: Yes Wed: No | C | N | N | Y | OS, recurrence, complications | ||||||
| Kilic et al. 2009 ‡ | USA | Retro | 2002–2007 | 50.0 | 75- | p I ≤ 7 cm (TNM v.6) | 106 | - | 78 | - | Lob/Seg: Yes | I, C | N | N | N | Y | Y | Y | N | Y | OS, DFS, recurrence, mortality, complications | |
| Ghosh et al. 2003 | UK | Retro | 1991–2001 | 42.3 | 70- | p T1N0 (TNM v.6) | 149 | - | - | 47 | Lob: Yes Wed: No | C | N | Y | N | OS, Mortality, complications | ||||||
| Outcomes | Comparison | No. of Studies | Certainty Assessment | Effect | Quality | Forest Plot | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | HR/OR (95% CI) | |||||
| OS 1 † | Seg vs. Lob | 3 [3,11] | Serious | Not serious | Not serious | Not serious | Undetected | 1.07 (0.98–1.18) | ⊕⊕⊕○ (Moderate) | Figure 2a |
| Wed vs. Lob | 3 [3,11] | Serious | Not serious | Not serious | Not serious | Undetected | 1.28 (1.22–1.35) | ⊕⊕⊕○ (Moderate) | Figure 2a | |
| OS 2 † | Seg vs. Lob | 5 [12,24,25,30,33] | Serious | Not serious | Not serious | Not serious | Undetected | 1.00 (0.78–1.27) | ⊕⊕⊕○ (Moderate) | Figure 2b |
| Sub vs. Lob | 10 [12,13,14,22,23,24,26,27,30,32] | Serious | Not serious | Not serious | Not serious | Undetected | 1.18 (0.97–1.43) | ⊕⊕⊕○ (Moderate) | Figure 2b | |
| Wed vs. Lob | 8 [12,22,23,24,29,30,34] | Serious | Not serious | Not serious | Not serious | Undetected | 1.13 (0.91–1.40) | ⊕⊕⊕○ (Moderate) | Figure 2b | |
| CSS | Seg vs. Lob | 8 [3,24,30] | Serious | Serious | Not serious | Not serious | Undetected | 1.01 (0.88–1.17) | ⊕⊕○ ○ (Low) | Figure 3a |
| Sub vs. Lob | 3 [3,24,30] | Serious | Serious | Not serious | Not serious | Undetected | 1.02 (0.93–1.12) | ⊕⊕○ ○ (Low) | Figure 3a | |
| Wed vs. Lob | 8 [3,24,30] | Serious | Serious | Not serious | Not serious | Undetected | 1.17 (1.06–1.30) | ⊕⊕○ ○ (Low) | Figure 3a | |
| DFS | Seg vs. Lob | 4 [12,24,25,33] | Serious | Not serious | Not serious | Not serious | Undetected | 1.04 (0.80–1.34) | ⊕⊕⊕○ (Moderate) | Figure 3b |
| Sub vs. Lob | 5 [12,13,14,23,24] | Serious | Not serious | Not serious | Not serious | Undetected | 1.07 (0.85–1.35) | ⊕⊕⊕○ (Moderate) | Figure 3b | |
| Wed vs. Lob | 4 [12,13,24] | Serious | Not serious | Not serious | Not serious | Undetected | 1.44 (1.01–2.05) | ⊕⊕⊕○ (Moderate) | Figure 3b | |
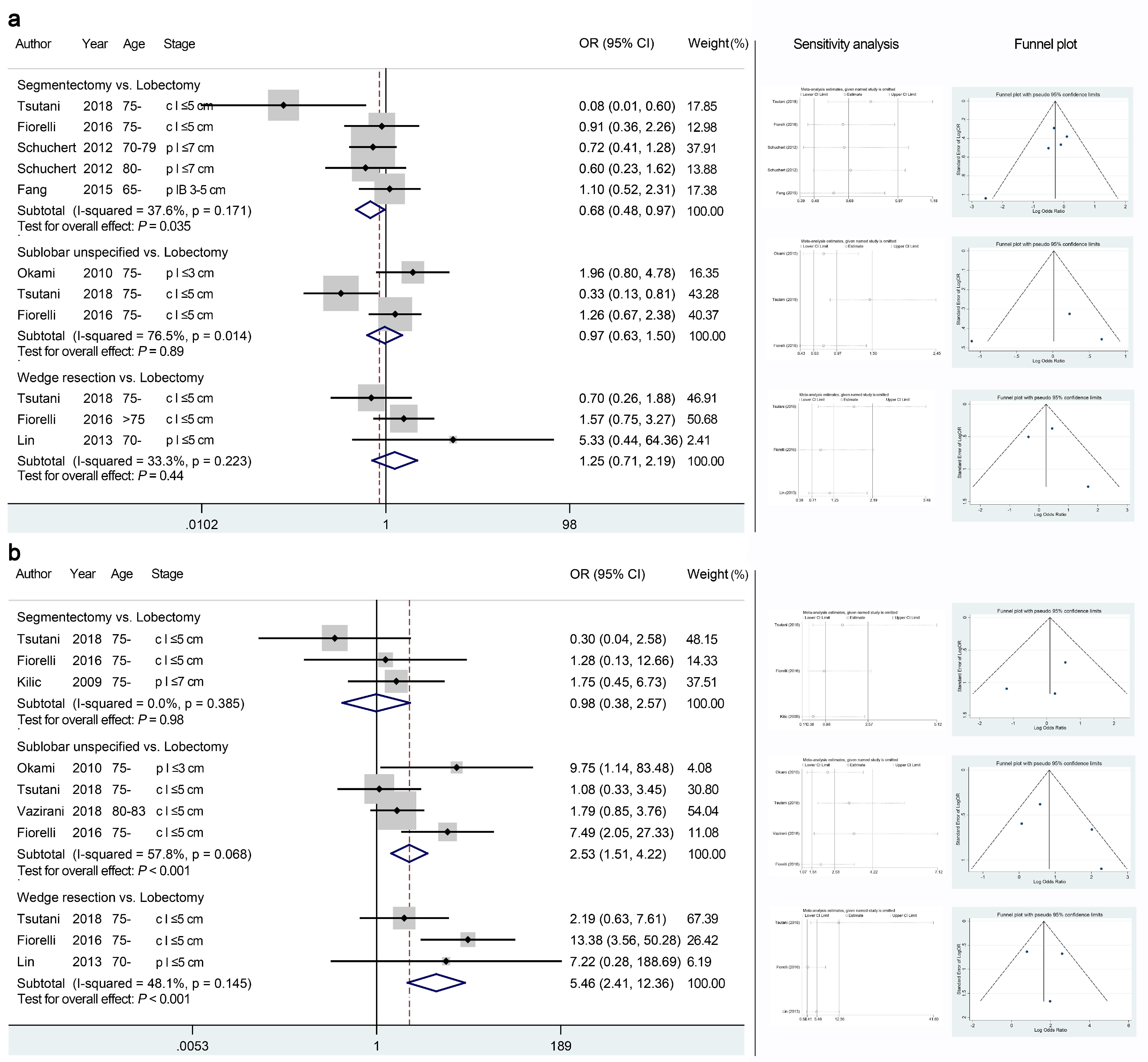
| Overall recurrence | Seg vs. Lob | 5 [14,24,25,31] | Serious | Not serious | Not serious | Not serious | Undetected | 0.68 (0.48–0.97) | ⊕⊕⊕○ (Moderate) | Figure 4a |
| Sub vs. Lob | 3 [14,24,32] | Serious | Serious | Not serious | Not serious | Undetected | 0.97 (0.63–1.50) | ⊕⊕○ ○ (Low) | Figure 4a | |
| Wed vs. Lob | 3 [14,24,28] | Serious | Not serious | Not serious | Not seri2295ous | Undetected | 1.25 (0.71–2.19) | ⊕⊕⊕○ (Moderate) | Figure 4a | |
| Local recurrence | Seg vs. Lob | 3 [14,24,33] | Serious | Not serious | Not serious | Not serious | Undetected | 0.98 (0.38–2.57) | ⊕⊕⊕○ (Moderate) | Figure 4b |
| Sub vs. Lob | 4 [14,22,24,32] | Serious | Not serious | Not serious | Not serious | Undetected | 2.53 (1.51–4.22) | ⊕⊕⊕○ (Moderate) | Figure 4b | |
| Wed vs. Lob | 3 [14,24,28] | Serious | Not serious | Not serious | Not serious | Undetected | 5.46 (2.41–12.4) | ⊕⊕⊕○ (Moderate) | Figure 4b | |
| Distant metastasis | Seg vs. Lob | 3 [14,24,33] | Serious | Not serious | Not serious | Not serious | Undetected | 0.49 (0.26–0.91) | ⊕⊕⊕○ (Moderate) | Sup-Figure S2 |
| Sub vs. Lob | 3 [14,24,32] | Serious | Serious | Not serious | Not serious | Undetected | 0.46 (0.26–0.81) | ⊕⊕○ ○ (Low) | Sup-Figure S2 | |
| Wed vs. Lob | 3 [14,24,28] | Serious | Not serious | Not serious | Not serious | Undetected | 0.30 (0.12–0.78) | ⊕⊕⊕○ (Moderate) | Sup-Figure S2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, S.; Liu, Z.; Sui, X.; Wang, X.; Li, X.; Qiu, M.; Yang, F. Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 294. https://doi.org/10.3390/jcm11020294
Wang P, Wang S, Liu Z, Sui X, Wang X, Li X, Qiu M, Yang F. Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(2):294. https://doi.org/10.3390/jcm11020294
Chicago/Turabian StyleWang, Peiyu, Shaodong Wang, Zheng Liu, Xizhao Sui, Xun Wang, Xiao Li, Mantang Qiu, and Fan Yang. 2022. "Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 2: 294. https://doi.org/10.3390/jcm11020294
APA StyleWang, P., Wang, S., Liu, Z., Sui, X., Wang, X., Li, X., Qiu, M., & Yang, F. (2022). Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(2), 294. https://doi.org/10.3390/jcm11020294






