The Development of Electroretinographic Oscillatory Potentials in Healthy Young Children
Abstract
1. Introduction
2. Methods
2.1. Ethical Guidelines
2.2. Subjects
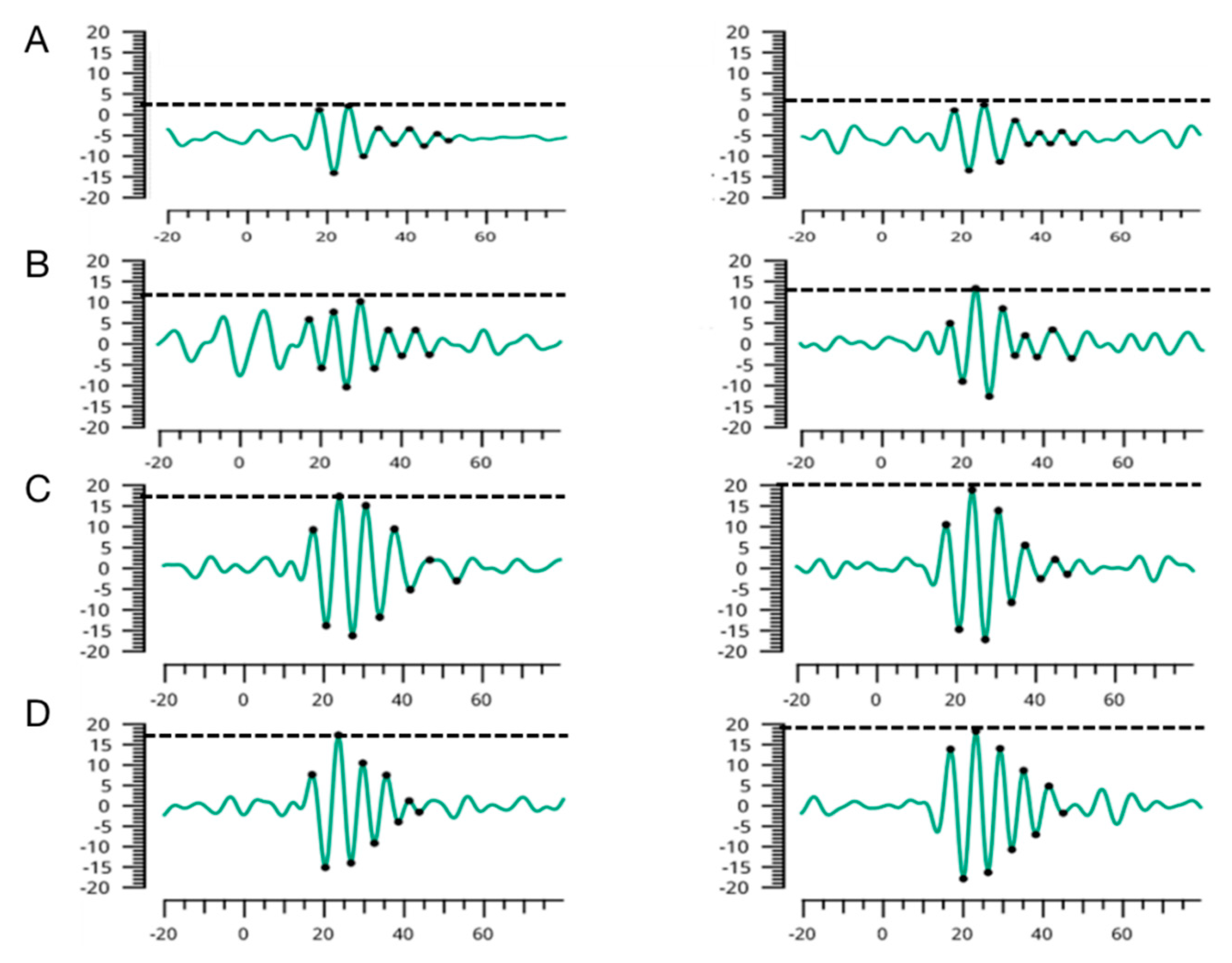
2.3. RETeval Electroretinographic Oscillatory Potentials
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the 132 Healthy Children
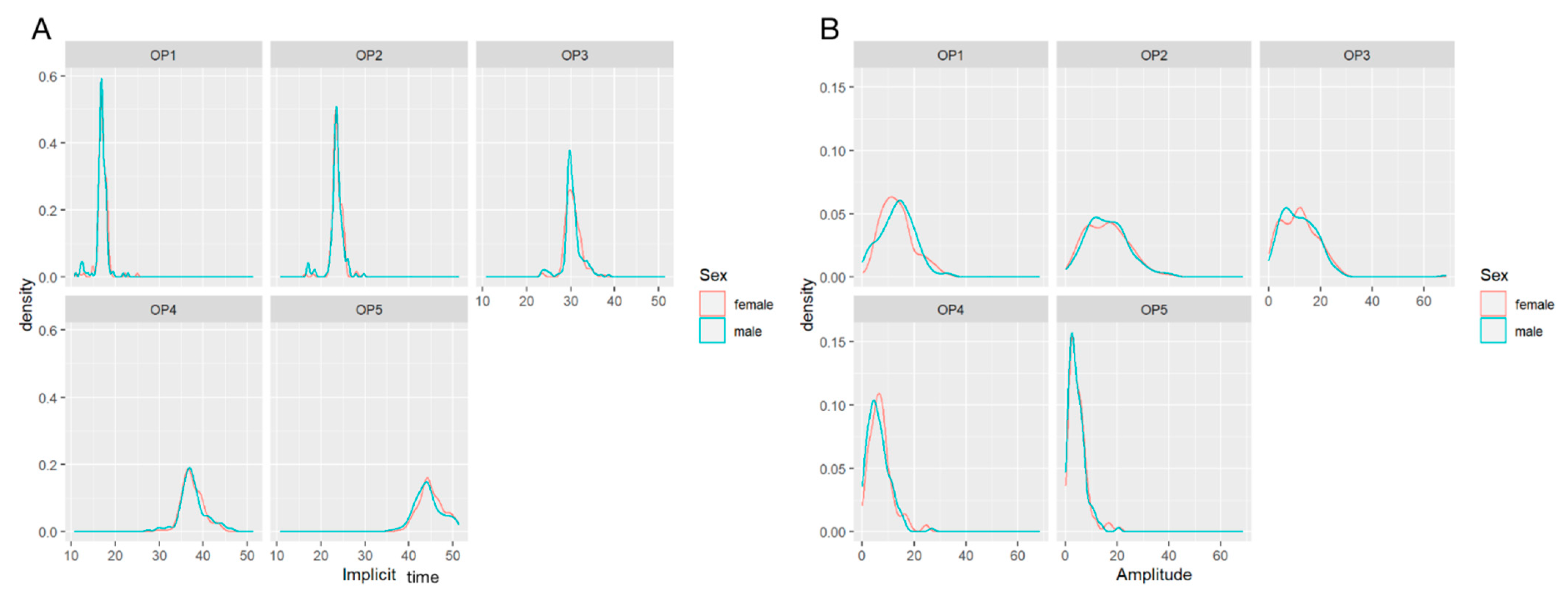
3.2. Comparison of Amplitudes and Implicit Times in OP1-5 by Gender
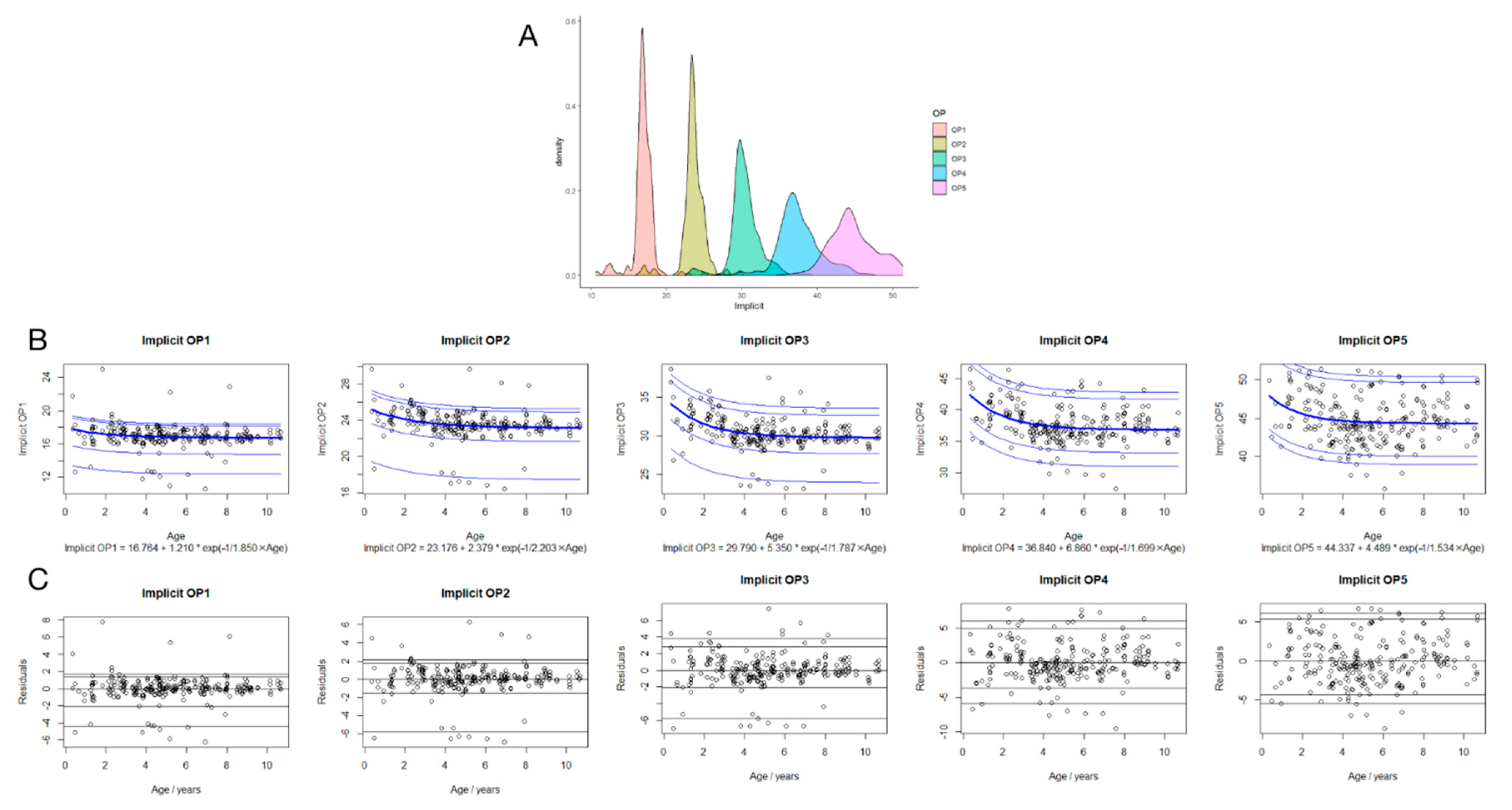
3.3. Regression Analysis between Implicit Times in OP1-5 and Age
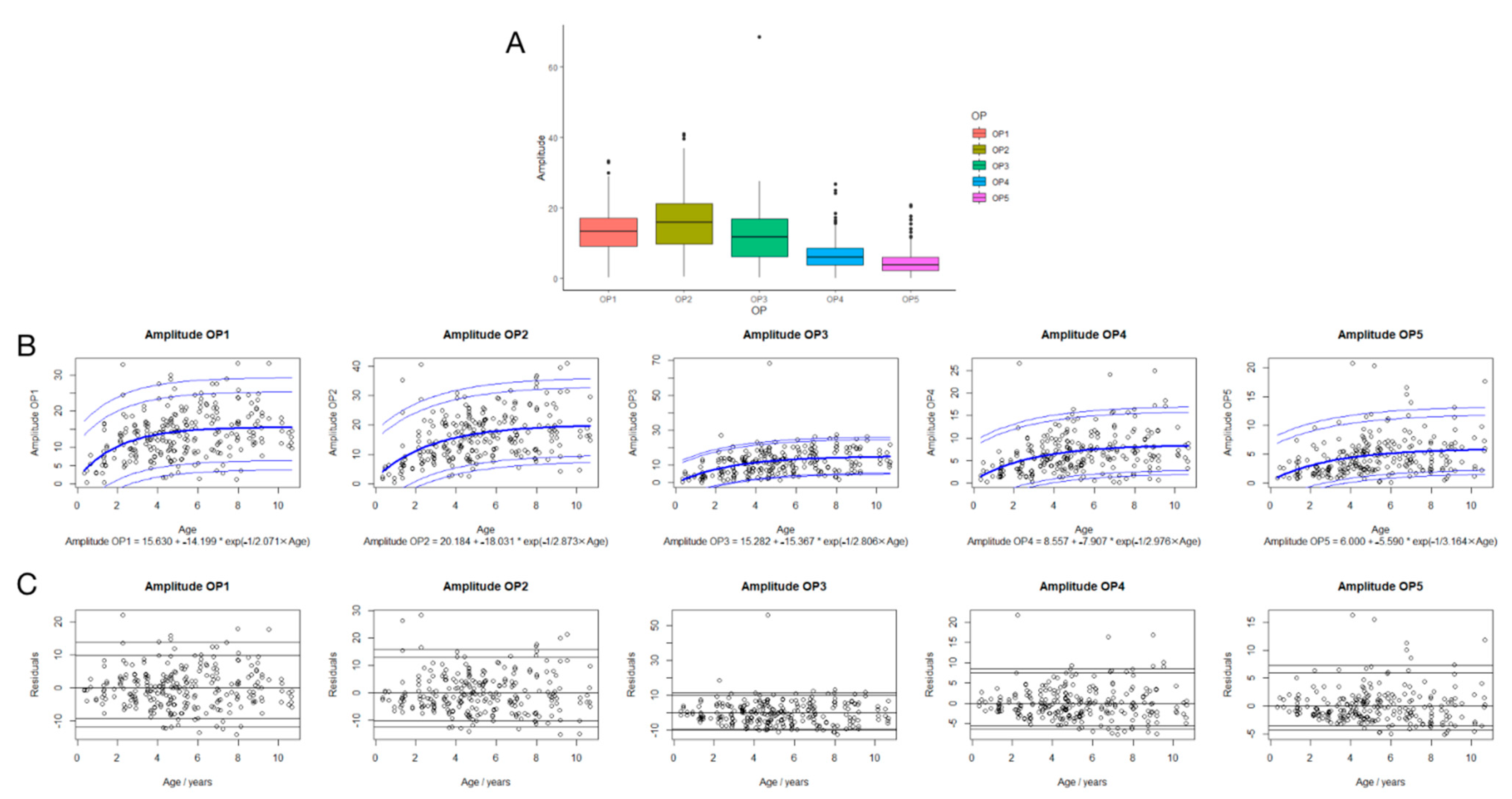
3.4. Regression Analysis between Amplitudes in OP1-5 and Age
3.5. Suggested Reference Data of Implicit Time and Amplitude of OP1-5 in Children
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motz, C.T.; Chesler, K.C.; Allen, R.S.; Bales, K.L.; Mees, L.M.; Feola, A.J.; Maa, A.Y.; Olson, D.E.; Thule, P.M.; Iuvone, P.M.; et al. Novel Detection and Restorative Levodopa Treatment for Preclinical Diabetic Retinopathy. Diabetes 2020, 69, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Agey, P.; Hare, W.A. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis. Neurosci. 2004, 21, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T. The electroretinogram: Its components and their origins. UCLA Forum Med. Sci. 1969, 8, 319–378. [Google Scholar] [CrossRef]
- Moller, A.; Eysteinsson, T. Modulation of the components of the rat dark-adapted electroretinogram by the three subtypes of GABA receptors. Vis. Neurosci. 2003, 20, 535–542. [Google Scholar] [CrossRef]
- Ogden, T.E. The oscillatory waves of the primate electroretinogram. Vis. Res. 1973, 13, 1059–1074. [Google Scholar] [CrossRef]
- Rangaswamy, N.V.; Hood, D.C.; Frishman, L.J. Regional variations in local contributions to the primate photopic flash ERG: Revealed using the slow-sequence mfERG. Investig. Ophthalmol. Vis. Res. 2003, 44, 3233–3247. [Google Scholar] [CrossRef]
- Wachtmeister, L. Some aspects of the oscillatory response of the retina. Prog. Brain Res. 2001, 131, 465–474. [Google Scholar]
- Moskowitz, A.; Hansen, R.M.; Fulton, A.B. ERG oscillatory potentials in infants. Doc. Ophthalmol. 2005, 110, 265–270. [Google Scholar] [CrossRef]
- Li, X.; Schaeffel, F.; Konrad, K.; Eberhart, Z. A dose related response of 6-OHDA on chicken spectral sensitivity and oscillatory potentials of recording electroretinograms. Chin. Med. J. 1996, 109, 762–770. [Google Scholar]
- Chen, J.C.; Brown, B.; Schmid, K.L. Evaluation of inner retinal function in myopia using oscillatory potentials of the multifocal electroretinogram. Vis. Res. 2006, 46, 4096–4103. [Google Scholar] [CrossRef]
- Luu, C.D.; Szental, J.A.; Lee, S.Y.; Lavanya, R.; Wong, T.Y. Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 482–486. [Google Scholar] [CrossRef]
- Holopigian, K.; Seiple, W.; Lorenzo, M.; Carr, R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2773–2780. [Google Scholar]
- Negretto, A.D.; Rosa, A.A.; Nakashima, A.A.; Ortega, K.C.; Mion, D., Jr.; Oyamada, M.K.; Nakashima, Y. Evaluation of hypertensive retinopathy through the oscillatory potentials of the electroretinogram. Arq. Bras. Oftalmol. 2008, 71, 38–42. [Google Scholar] [CrossRef][Green Version]
- Holopigian, K.; Greenstein, V.C.; Seiple, W.; Hood, D.C.; Ritch, R. Electrophysiologic assessment of photoreceptor function in patients with primary open-angle glaucoma. J. Glaucoma 2000, 9, 163–168. [Google Scholar] [CrossRef]
- Hara, A.; Miura, M. Decreased inner retinal activity in branch retinal vein occlusion. Doc. Ophthalmol. 1994, 88, 39–47. [Google Scholar] [CrossRef]
- Walter, P.; Widder, R.A.; Luke, C.; Königsfeld, P.; Brunner, R. Electrophysiological abnormalities in age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 962–968. [Google Scholar] [CrossRef]
- Westall, C.A.; Panton, C.M.; Levin, A.V. Time courses for maturation of electroretinogram responses from infancy to adulthood. Doc. Ophthalmol. 1998, 96, 355–379. [Google Scholar] [CrossRef]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Lachapelle, P.; Benoit, J. Interpretation of the filtered 100- to 1000-Hz electroretinogram. Doc. Ophthalmol. 1994, 86, 33–46. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, J.; Sun, L.; Li, S.; Huang, L.; Wang, Y.; Li, Z.; Cao, L.; Ding, X. Mydriasis-Free Flicker Electroretinograms in 204 Healthy Children Aged 0–18 Years: Reference Data from Two Cohorts. Transl. Vis. Sci. Technol. 2021, 10, 7. [Google Scholar] [CrossRef]
- Maa, A.Y.; Feuer, W.J.; Davis, C.Q.; Pillow, E.K.; Brown, T.D.; Caywood, R.M.; Chasan, J.E.; Fransen, S.R. A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J. Diabetes Complicat. 2016, 30, 524–532. [Google Scholar] [CrossRef]
- Fukuo, M.; Kondo, M.; Hirose, A.; Fukushima, H.; Ikesugi, K.; Sugimoto, M.; Kato, K.; Uchigata, Y.; Kitano, S. Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci. Rep. 2016, 6, 36591. [Google Scholar] [CrossRef]
- Davis, C.Q.; Hamilton, R. Reference ranges for clinical electrophysiology of vision. Doc. Ophthalmol. 2021, 143, 155–170. [Google Scholar] [CrossRef]
- Wan, W.; Chen, Z.; Lei, B. Increase in electroretinogram rod-driven peak frequency of oscillatory potentials and dark-adapted responses in a cohort of myopia patients. Doc. Ophthalmol. 2020, 140, 189–199. [Google Scholar] [CrossRef]
- Sannita, W.G.; Maggi, L.; Germini, P.L.; Fioretto, M. Correlation with age and sex of flash-evoked electroretinogram and retinal oscillatory potentials recorded with skin electrodes. Doc. Ophthalmol. 1989, 71, 413–419. [Google Scholar] [CrossRef]
- Parness-Yossifon, R.; Mets, M.B. The electroretinogram in children. Curr. Opin. Ophthalmol. 2008, 19, 398–402. [Google Scholar] [CrossRef]
- Mactier, H.; Bradnam, M.S.; Hamilton, R. Dark-adapted oscillatory potentials in preterm infants with and without retinopathy of prematurity. Doc. Ophthalmol. 2013, 127, 33–40. [Google Scholar] [CrossRef]
| Median, Range | ||||
|---|---|---|---|---|
| Male | Female | p | ||
| Implicit time (ms) | ||||
| OP1 | 16.9 (11.0–22.9) | 17.0 (10.6–25.0) | 0.180 | |
| OP2 | 23.5 (16.8–29.7) | 23.6 (16.4–29.7) | 0.185 | |
| OP3 | 30.1 (23.3–38.6) | 30.5 (23.2–37.5) | 0.625 | |
| OP4 | 37.3 (27.4–46.5) | 37.3 (29.6–44.2) | 0.963 | |
| OP5 | 44.0 (35.8–51.2) | 44.5 (37.8–51.4) | 0.083 | |
| Amplitude (μV) | ||||
| OP1 | 13.9 (0.3–33.1) | 12.0 (0.4–33.2) | 0.975 | |
| OP2 | 15.0 (0.5–40.5) | 16.0 (2.0–41.0) | 0.803 | |
| OP3 | 11.5 (0.4–27.0) | 11.5 (0.2–27.5) | 0.727 | |
| OP4 | 3.7 (0.2–20.4) | 3.9 (0.1–20.8) | 0.100 | |
| OP5 | 5.3 (0.1–26.7) | 6.3 (0.3–24.2) | 0.318 | |
| Implicit Times (in ms) | Amplitudes (in µV) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.5% | 5.0% | 95.0% | 97.5% | 2.5% | 5.0% | 95.0% | 97.5% | |
| OP1 | −4.38 | −2.02 | 1.37 | 1.63 | −11.79 | −9.20 | 9.76 | 13.64 |
| OP2 | −5.76 | −1.56 | 1.73 | 2.12 | −12.47 | −10.25 | 12.99 | 16.02 |
| OP3 | −5.78 | −2.07 | 2.83 | 3.79 | −9.92 | −9.35 | 9.92 | 11.30 |
| OP4 | −5.93 | −3.70 | 4.87 | 5.95 | −6.42 | −5.53 | 7.48 | 8.63 |
| OP5 | −5.42 | −4.31 | 5.31 | 6.06 | −4.31 | −3.59 | 5.93 | 7.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Lu, J.; Jiang, Z.; Huang, L.; Zeng, J.; Cao, L.; Luo, X.; Yu, B.; Ding, X. The Development of Electroretinographic Oscillatory Potentials in Healthy Young Children. J. Clin. Med. 2022, 11, 5967. https://doi.org/10.3390/jcm11195967
Zhang T, Lu J, Jiang Z, Huang L, Zeng J, Cao L, Luo X, Yu B, Ding X. The Development of Electroretinographic Oscillatory Potentials in Healthy Young Children. Journal of Clinical Medicine. 2022; 11(19):5967. https://doi.org/10.3390/jcm11195967
Chicago/Turabian StyleZhang, Ting, Jinglin Lu, Zhaoxin Jiang, Li Huang, Jun Zeng, Liming Cao, Xiaoling Luo, Bilin Yu, and Xiaoyan Ding. 2022. "The Development of Electroretinographic Oscillatory Potentials in Healthy Young Children" Journal of Clinical Medicine 11, no. 19: 5967. https://doi.org/10.3390/jcm11195967
APA StyleZhang, T., Lu, J., Jiang, Z., Huang, L., Zeng, J., Cao, L., Luo, X., Yu, B., & Ding, X. (2022). The Development of Electroretinographic Oscillatory Potentials in Healthy Young Children. Journal of Clinical Medicine, 11(19), 5967. https://doi.org/10.3390/jcm11195967






