Epidemiological Study of Lumbar Spinal Stenosis Symptoms: 10-Year Follow-Up in the Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
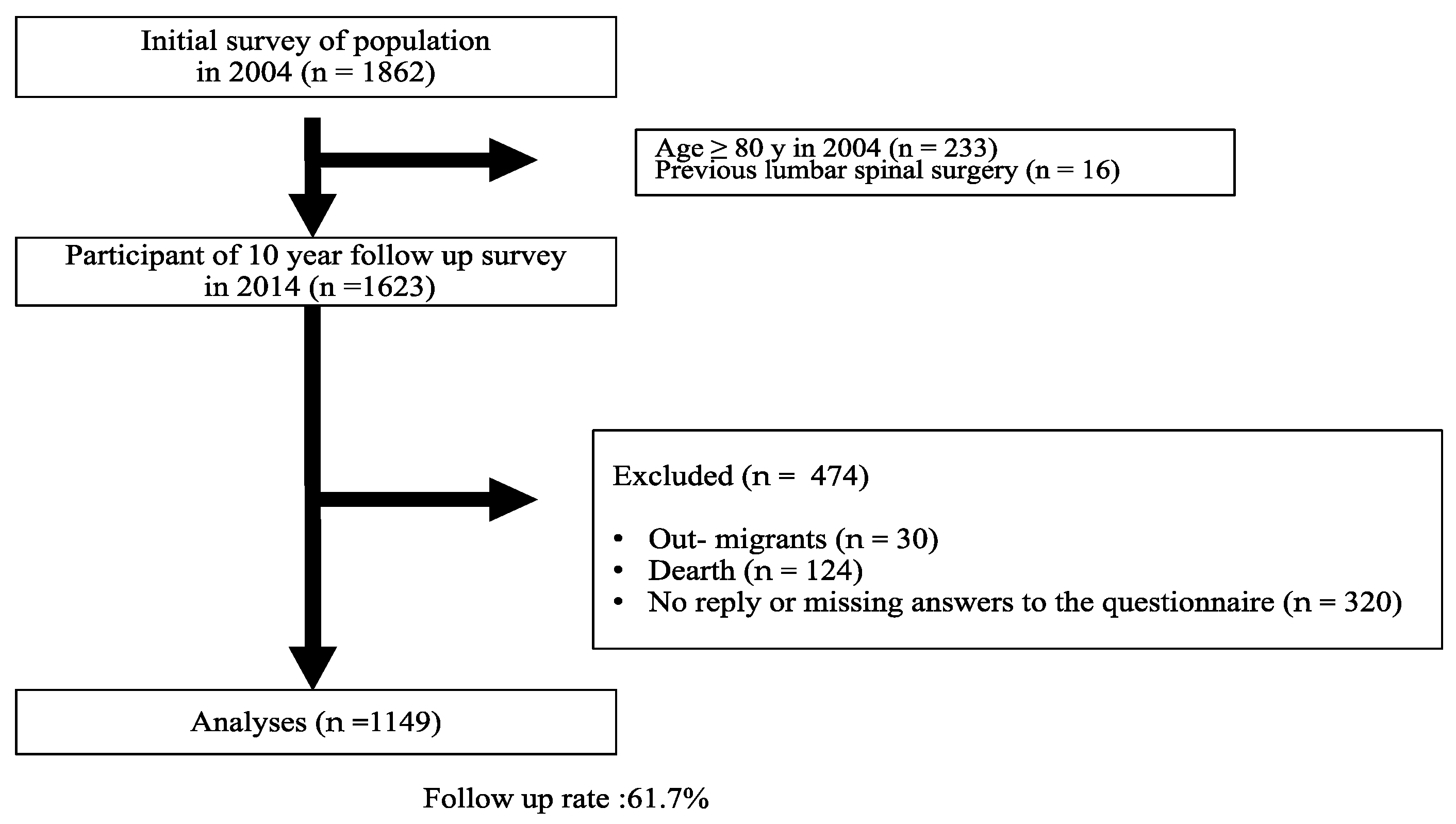
2.2. Participants
2.3. Diagnosis of LSS Symptoms
2.4. QOL Evaluation
2.5. Predictive Factors of the Presence of LSS Symptoms at 10-Year Follow-Up
2.6. Statistical Analysis
3. Results
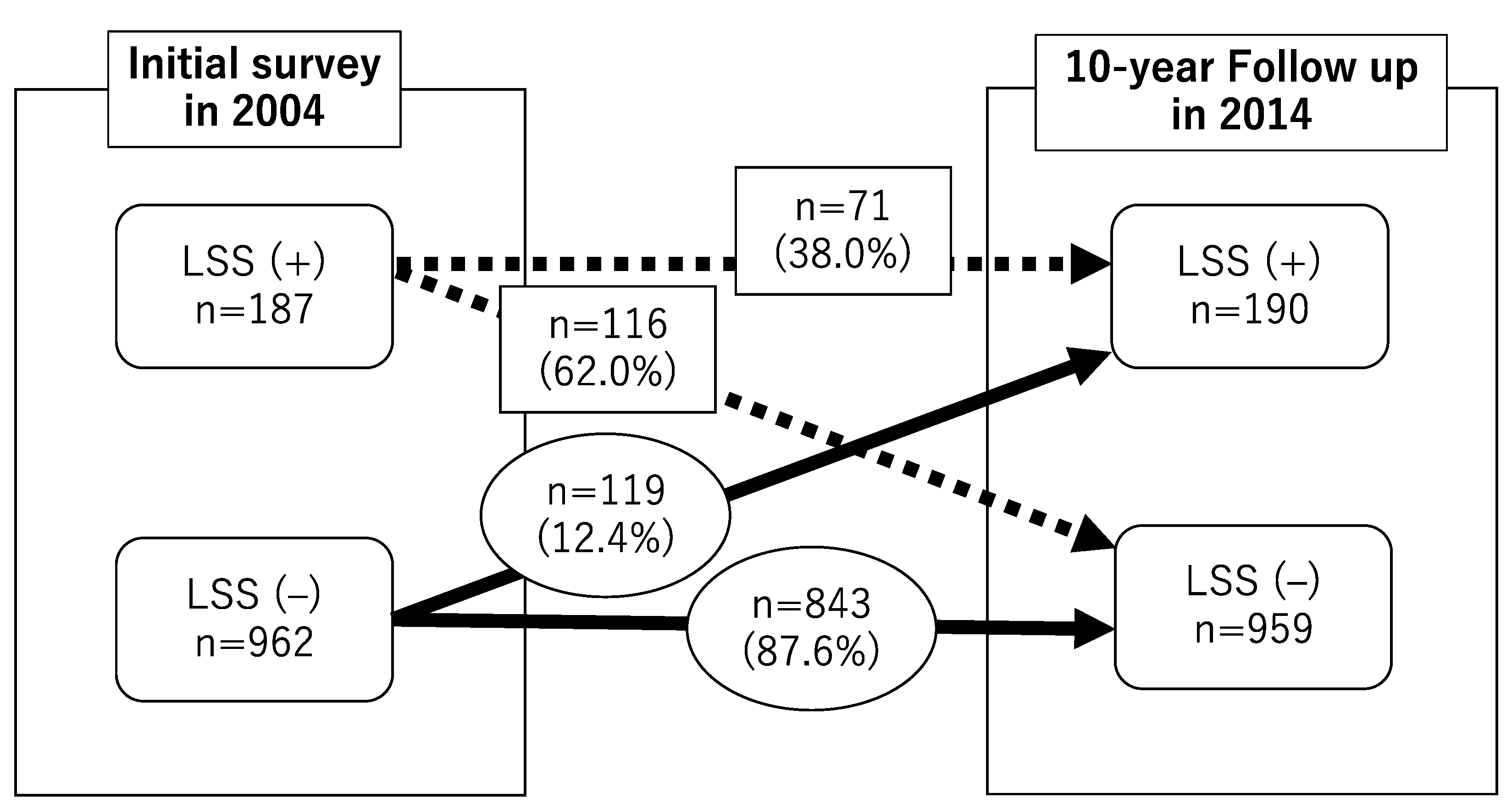
3.1. Time Course of LSS Symptoms
3.2. Relationship between LBP-Related QOL and the Time Course of LSS Symptoms
3.3. Relationship between HR-QOL and the Time Course of LSS
3.4. Predictive Factor of the Presence of LSS Symptoms during the 10-Year Follow-Up Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Self-Administered, Self-Reported History Questionnaire for LSS(LSS-SSHQ) [11]
References
- Katz, J.N.; Harris, M.B. Clinical practice. Lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 818–825. [Google Scholar] [CrossRef]
- Otani, K.; Kikuchi, S.; Yabuki, S.; Igarashi, T.; Nikaido, T.; Watanabe, K.; Konno, S. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: An epidemiological cross-sectional Study of 1862 community-dwelling individuals. Sci. World J. 2013, 23, 590652. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Fukumori, N.; Takegami, M.; Onishi, Y.; Otani, K.; Sekiguchi, M.; Wakita, T.; Kikuchi, S.; Fukuhara, S.; Konno, S. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: A population-based study. J. Orthop. Sci. 2013, 18, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, D. Preoperative health-related quality of life and associated factors in lumbar spinal stenosis. J. Spine Res. 2012, 3, 159–165. [Google Scholar]
- Amundsen, T.; Weber, H.; Lilleas, F.; Nordal, H.J.; Abdelnoor, M.; Magnaes, B. Lumbar spinal stenosis: Clinical and radiologic features. Spine 1995, 20, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Dalgas, M.; Stucki, G.; Katz, N.P.; Bayley, J.; Fossel, A.H.; Chang, L.C.; Lipson, S.J. Degenerative lumbar spinal stenosis: Diagnostic value of the history and physical examination. Arthritis Rheum. 1995, 38, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Geisser, M.E.; Haig, A.J.; Tong, H.C.; Yamakawa, K.S.; Quint, D.J.; Hoff, J.T.; Miner, J.; Phalke, V.V. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin. J. Pain 2007, 9, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Zeifang, F.; Schiltenwolf, M.; Abel, R.; Moradi, B. Gait analysis does not correlate with clinical and MR imaging parameters in patients with symptomatic lumbar spinal stenosis. BMC Musculoskelet. Disord. 2008, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Kikuchi, S.; Yabuki, S.; Onda, A.; Nikaido, T.; Watanabe, K.; Konno, S. Prospective one-year follow-up of lumbar spinal stenosis in a regional community. J. Pain Res. 2018, 11, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Kikuchi, S.; Yabuki, S.; Nikaido, T.; Watanabe, K.; Kato, K.; Kobayashi, H.; Konno, S. The Change of Lumbar Spinal Stenosis Symptoms over a Six-Year Period in Community-Dwelling People. Medicina 2021, 57, 1116. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Kikuchi, S.; Tanaka, Y.; Yamazaki, K.; Shimada, Y.; Takei, H.; Yokoyama, T.; Okada, M.; Kokubun, S. A diagnostic support tool for lumbar spinal stenosis: A self-administered, self-reported history questionnaire. BMC Musculoskelet. Disord. 2007, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Fukuhara, S.; Ware, J.E., Jr.; Kosinski, M.; Wada, B.; Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1045–1053. [Google Scholar] [CrossRef]
- Fukuhara, S.; Suzukamo, Y.; Bito, S.; Kurokawa, K. Manual of SF-36 Japanese Version 1.2; Public Health Research Foundation: Tokyo, Japan, 2001. [Google Scholar]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Suzukamo, Y.; Fukuhara, S.; Kikuchi, S.; Konno, S.; Roland, M.; Iwamoto, Y.; Nakamura, T. Validation of the Japanese version of the Roland-Morris Disability Questionnaire. J. Orthop. Sci. 2003, 8, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S. Manual of RDQ Japanese Version Low Back Pain-Related QoL; Iryoubunkasya: Tokyo, Japan, 2004. [Google Scholar]
- Yamazaki, S.; Fukuhara, S.; Green, J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual. Life Outcomes 2005, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Minamide, A.; Yoshida, M.; Maio, K. The natural clinical course of lumbar spinal stenosis: A longitudinal cohort study over a minimum of 10 years. J. Orthop. Sci. 2013, 18, 693–698. [Google Scholar] [CrossRef] [PubMed]
- North American Spine Society. Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care, Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis; North American Spine Society: Burr Ridge, IL, USA, 2011. [Google Scholar]
- Uesugi, K.; Sekiguchi, M.; Kikuchi, S.; Konno, S. Relationship between lumbar spinal stenosis and lifestyle-related disorders: A cross-sectional multicenter observational study. Spine 2013, 38, E540–E545. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 1149) | Number of Missing Data | |
|---|---|---|
| Age (y) (mean ± SD) | 63.0 ± 11.6 | 0 |
| Age in 2004 (n [%]) | 0 | |
| ≤49 | 155 (13.5) | |
| 50–59 | 202 (17.6) | |
| 60–69 | 414 (36.0) | |
| 70–79 | 378 (32.9) | |
| Sex (n [%]) | 0 | |
| Male | 406 (35.3) | |
| Female | 743 (64.7) | |
| BMI (kg/m2) (n [%]) | 79 | |
| <18.5 | 32 (30.0) | |
| ≥18.5, <25 | 692 (64.7) | |
| ≥25, <30 | 313 (29.3) | |
| ≥30 | 33 (3.1) | |
| Prevalence of LSS (n [%]) | 189 (16.4) | 0 |
| Smoking (n [%]) | 205 (19.2) | 79 |
| Comorbidities (n [%]) | 79 | |
| Respiratory | 3 (0.3) | |
| Diabetes mellitus | 54 (5.0) | |
| Cardiovascular | 83 (7.8) | |
| Cerebrovascular | 8 (0.7) | |
| Hypertension | 358 (33.5) | |
| Depressive symptoms (MHI-5) (n [%]) | 85 | |
| None | 726 (68.2) | |
| Mild | 121 (11.4) | |
| Moderate | 96 (9.0) | |
| Severe | 122 (11.5) | |
| RDQ standardized score (n [%]) | 22 | |
| ≥50 | 890 (79.0) | |
| <50 | 237 (21.0) | |
| Prevalence of Knee OA (n [%]) | 303 (28.4) | 83 |
| Prevalence of Hip OA (n [%]) | 59 (5.2) | 5 |
| Time Course of LSS Symptoms from 2004 to 2014 | |||||
|---|---|---|---|---|---|
| Age in 2004 (years) | (+) → (+) n = 71 | (+) → (−) n = 116 | (−) → (+) n = 119 | (−) → (−) n = 843 | n |
| ≦ 49 | 0 (0) | 4 (2.6) | 9 (5.8) | 142 (91.6) | 155 |
| 50–59 | 7 (3.5) | 15 (7.4) | 17 (8.4) | 163 (80.7) | 202 |
| 60–69 | 26 (6.3) | 45 (10.9) | 48 (11.6) | 295 (71.3) | 414 |
| 70–79 | 38 (10.1) | 52 (13.8) | 45 (11.9) | 243 (64.3) | 378 |
| Time Course of LSS | RDQ Norm-Based Score | ||
|---|---|---|---|
| Initial Survey | 10-Year Follow-Up | p Value | |
| (+) → (+) | 48.2 ± 8.4 | 51.4 ± 9.8 | 0.067 |
| (+) → (−) | 49.5 ± 9.5 | 55.9 ± 9.1 | <0.0001 |
| (−) → (+) | 53.0 ± 9.0 | 51.1 ± 10.6 | 0.3897 |
| (−) → (−) | 56.1 ± 6.3 | 58.4 ± 5.1 | <0.0001 |
| (+) → (+) | 48.2 ± 8.4 | 51.4 ± 9.8 | 0.067 |
| Time Course of LSS Symptoms | Norm-Based Score | p Value | ||
|---|---|---|---|---|
| Initial Survey | 10-Year Follow-Up | |||
| PF | (+) → (+) | 47.9 ± 13.8 | 24.7 ± 22.9 | 0.0002 |
| (+) → (−) | 48.4 ± 10.3 | 36.9 ± 19.4 | 0.0017 | |
| (−) → (+) | 47.9 ± 15.6 | 31.7 ± 17.2 | 0.0013 | |
| (−) → (−) | 50.8 ± 11.7 | 45.1 ± 14.7 | <0.0001 | |
| RP | (+) → (+) | 43.4 ± 11.9 | 26.9 ± 15.2 | 0.0008 |
| (+) → (−) | 46.9 ± 11.8 | 37.2 ± 18.1 | 0.0122 | |
| (−) → (+) | 44.8 ± 13.1 | 39.1 ± 17.1 | 0.0484 | |
| (−) → (−) | 50.0 ± 10.0 | 45.1 ± 13.2 | <0.0001 | |
| BP | (+) → (+) | 47.7 ± 8.7 | 38.0 ± 8.8 | 0.0008 |
| (+) → (−) | 43.2 ± 7.6 | 42.1 ± 9.2 | 0.5483 | |
| (−) → (+) | 48.4 ± 10.1 | 35.9 ± 7.7 | <0.0001 | |
| (−) → (−) | 50.5 ± 10.1 | 46.8 ± 10.8 | <0.0001 | |
| GH | (+) → (+) | 45.7 ± 11.6 | 44.4 ± 7.2 | 0.5559 |
| (+) → (−) | 44.8 ± 8.3 | 47.6 ± 12.8 | 0.0243 | |
| (−) → (+) | 48.6 ± 9.6 | 44.2 ± 6.7 | 0.1717 | |
| (−) → (−) | 51.3 ± 9.2 | 51.3 ± 9.5 | 0.2334 | |
| VT | (+) → (+) | 51.7 ± 6.8 | 44.2 ± 7.0 | 0.0092 |
| (+) → (−) | 49.5 ± 9.7 | 48.2 ± 11.5 | 0.4245 | |
| (−) → (+) | 49.4 ± 11.5 | 45.3 ± 8.7 | 0.0884 | |
| (−) → (−) | 53.1 ± 8.7 | 50.7 ± 9.5 | 0.0094 | |
| SF | (+) → (+) | 49.1 ± 10.0 | 39.0 ± 13.7 | <0.0001 |
| (+) → (−) | 47.7 ± 11.7 | 46.0 ± 15.0 | 0.5749 | |
| (−) → (+) | 48.1 ± 11.1 | 41.7 ± 11.4 | 0.0446 | |
| (−) → (−) | 50.8 ± 9.3 | 48.8 ± 11.2 | 0.0049 | |
| RE | (+) → (+) | 49.9 ± 10.7 | 32.7 ± 15.3 | 0.0009 |
| (+) → (−) | 46.9 ± 12.4 | 42.5 ± 17.1 | 0.2191 | |
| (−) → (+) | 45.2 ± 13.9 | 35.5 ± 11.3 | 0.0123 | |
| (−) → (−) | 50.7 ± 9.6 | 46.7 ± 12.7 | <0.0001 | |
| MH | (+) → (+) | 46.7 ± 12.9 | 45.1 ± 7.9 | 0.6228 |
| (+) → (−) | 45.7 ± 9.6 | 47.2 ± 10.9 | 0.4428 | |
| (−) → (+) | 46.6 ± 9.8 | 43.8 ± 9.3 | 0.0889 | |
| (−) → (−) | 50.4 ± 9.2 | 51.3 ± 9.9 | 0.0841 | |
| Model 1 (n = 1149) | Model 2 (n = 730) | ||||||
|---|---|---|---|---|---|---|---|
| Category 1 | Category 2 | OR | 95%CI | p Value | OR | 95%CI | p Value |
| Gender | Female | 0.841 | 0.546–1.295 | 0.4315 | 1.07 | 0.627–1.826 | 0.8701 |
| Age (years) | ≤49 | 0.623 | 0.257–1.511 | 0.295 | 0.615 | 0.230–1.644 | 0.3322 |
| 50–59 | reference | reference | |||||
| 60–69 | 1.437 | 0.803–2.751 | 0.2221 | 1.672 | 0.809–3.455 | 0.1654 | |
| 70–79 | 1.696 | 0.943–3.051 | 0.0777 | 1.673 | 0.776–3.607 | 0.1891 | |
| BMI | <18.5 | reference | reference | ||||
| 18.5–24.9 | 4.565 | 0.993–20.975 | 0.0551 | 3.2 × 108 | - | 0.9981 | |
| 25.0–29.9 | 3.929 | 0.838–18.428 | 0.0826 | 3.4 × 108 | - | 0.9981 | |
| 30.0≤ | 3.044 | 0.426–2.764 | 0.2673 | 1.2 × 108 | - | 0.9982 | |
| RDQ norm-based score | 50≤ | reference | reference | ||||
| <50 | 1.892 | 1.230–2.910 | 0.0037 | 1.405 | 0.699–2.824 | 0.3404 | |
| Knee OA | Positive | 1.984 | 1.302–2.964 | 0.013 | |||
| Hip OA | Positive | 1.480 | 0.699–3.135 | 0.3056 | |||
| Comorbidities | Respiratory | 1.706 | 0.093–31.452 | 0.7193 | 2.7 × 10−9 | - | 0.9993 |
| Diabetes Mellitus | 1.345 | 0.627–2.886 | 0.4464 | 0.839 | 0.232–3.033 | 0.7883 | |
| Cardiovascular | 0.589 | 0.295–1.176 | 0.1335 | 0.581 | 0.159–2.216 | 0.4116 | |
| Cerebrovascular | 0.661 | 0.0072–6.088 | 0.7151 | 2.7 × 10−9 | - | 0.9993 | |
| Hypertension | |||||||
| Smoking | Pack-years | 1.000 | 1.000–1.001 | 0.2164 | 1.001 | 1.000–1.001 | 0.0187 |
| Depressive symptoms | None | reference | reference | ||||
| Mild | 1.534 | 0.885–2.661 | 0.1372 | 1.531 | 0.719–3.260 | 0.2688 | |
| Moderate | 0.809 | 0.389–1.681 | 0.570 | 0.949 | 0.346–2.603 | 0.919 | |
| Severe | 2.379 | 1.415–3.999 | 0.0011 | 3.487 | 1.709–7.113 | 0.0006 | |
| LSS symptoms in the initial survey | Positive | 2.749 | 1.765–4.282 | <0.0001 | 3.732 | 1.758–7.802 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igari, T.; Otani, K.; Sekiguchi, M.; Konno, S.-i. Epidemiological Study of Lumbar Spinal Stenosis Symptoms: 10-Year Follow-Up in the Community. J. Clin. Med. 2022, 11, 5911. https://doi.org/10.3390/jcm11195911
Igari T, Otani K, Sekiguchi M, Konno S-i. Epidemiological Study of Lumbar Spinal Stenosis Symptoms: 10-Year Follow-Up in the Community. Journal of Clinical Medicine. 2022; 11(19):5911. https://doi.org/10.3390/jcm11195911
Chicago/Turabian StyleIgari, Takahiro, Koji Otani, Miho Sekiguchi, and Shin-ichi Konno. 2022. "Epidemiological Study of Lumbar Spinal Stenosis Symptoms: 10-Year Follow-Up in the Community" Journal of Clinical Medicine 11, no. 19: 5911. https://doi.org/10.3390/jcm11195911
APA StyleIgari, T., Otani, K., Sekiguchi, M., & Konno, S.-i. (2022). Epidemiological Study of Lumbar Spinal Stenosis Symptoms: 10-Year Follow-Up in the Community. Journal of Clinical Medicine, 11(19), 5911. https://doi.org/10.3390/jcm11195911






