Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials
Abstract
1. Introduction
2. Methods
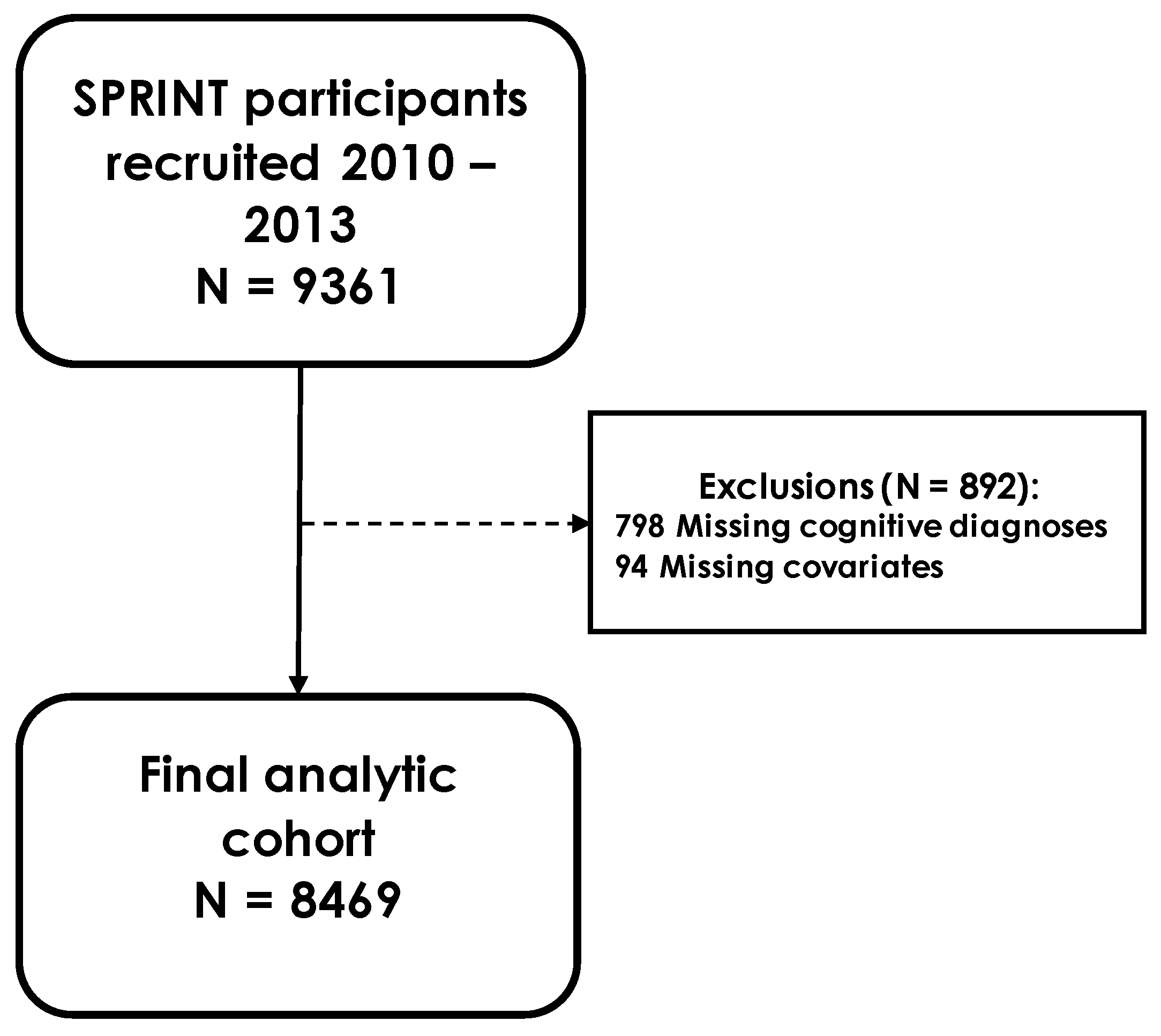
2.1. Study Population
2.2. Covariates
2.3. Atrial Fibrillation
2.4. Ascertainment of Mild Cognitive Impairment and Probable Dementia
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letsas, K.P.; Karamichalakis, N.; Vlachos, K.; Georgopoulos, S.; Bakalakos, A.; Efremidis, M.; Sideris, A. Managing atrial fibrillation in the very elderly patient: Challenges and solutions. Vasc. Health Risk Manag. 2015, 11, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kistler, P.M.; Sanders, P.; Fynn, S.P.; Stevenson, I.H.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J. Am. Coll. Cardiol. 2004, 44, 109–116. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S. Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; de Larriva, A.P.A. Atrial Fibrillation, Cognitive Decline And Dementia. Eur. Cardiol. 2016, 11, 49–53. [Google Scholar] [CrossRef]
- Dzeshka, M.S.; Shantsila, A.; Shantsila, E.; Lip, G.Y.H. Atrial Fibrillation and Hypertension. Hypertension 2017, 70, 854–861. [Google Scholar] [CrossRef]
- Iadecola, C. Hypertension and Dementia. Hypertension 2014, 64, 3–5. [Google Scholar] [CrossRef] [PubMed]
- SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Ambrosius, W.T.; Sink, K.; Foy, C.G.; Berlowitz, D.; Cheung, A.K.; Cushman, W.C.; Fine, L.J.; Goff, J.D.C.; Johnson, K.C.; Killeen, A.A.; et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin. Trials 2014, 11, 532–546. [Google Scholar] [CrossRef]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; B D’Agostino, R.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; J O’Donnell, C.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Ambrosius, W.T.; Cushman, W.C.; Zhang, Z.M.; Bates, J.T.; Neyra, J.A.; Carson, T.Y.; Tamariz, L.; Ghazi, L.; Cho, M.E.; et al. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation 2017, 136, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Sprint Mind Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019, 321, 553–561. [Google Scholar] [CrossRef]
- Kawas, C.; Segal, J.; Stewart, W.F.; Corrada, M.; Thal, L.J. A Validation Study of the Dementia Questionnaire. Arch. Neurol. 1994, 51, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Norby, F.L.; Gottesman, R.F.; Mosley, T.H.; Soliman, E.Z.; Agarwal, S.K.; Loehr, L.R.; Folsom, A.R.; Coresh, J.; Alonso, A. Association of Atrial Fibrillation With Cognitive Decline and Dementia Over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J. Am. Heart Assoc. 2018, 7, e007301. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Knopman, D.S.; Gottesman, R.F.; Soliman, E.Z.; Shah, A.J.; O’Neal, W.T.; Norby, F.L.; Mosley, T.H.; Chen, L.Y. Correlates of Dementia and Mild Cognitive Impairment in Patients With Atrial Fibrillation: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J. Am. Heart Assoc. 2017, 6, e006014. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; Ferranti, S.d.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.R.; Beiser, A.S.; DeCarli, C.; Plourde, K.L.; Vasan, R.S.; Greenberg, S.M.; Seshadri, S. Blood pressure from mid- to late life and risk of incident dementia. Neurology 2017, 89, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Howard, G.; Meschia, J.F.; Cushman, M.; Muntner, P.; Pullicino, P.M.; McClure, L.A.; Judd, S.; Howard, V.J. Self-Reported Atrial Fibrillation and Risk of Stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke 2011, 42, 2950–2953. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Valzania, C.; Biffi, M.; Diemberger, I.; Ziacchi, M.; Martignani, C. Asymptomatic lone atrial fibrillation—How can we detect the arrhythmia? Curr. Pharm. Des. 2015, 21, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef]
- Boriani, G.; Laroche, C.; Diemberger, I.; Fantecchi, E.; Popescu, M.I.; Rasmussen, L.H.; Sinagra, G.; Petrescu, L.; Tavazzi, L.; Maggioni, A.P.; et al. Asymptomatic Atrial Fibrillation: Clinical Correlates, Management, and Outcomes in the EORP-AF Pilot General Registry. Am. J. Med. 2015, 128, 509–518.e2. [Google Scholar] [CrossRef]
- Frykman, V.; Frick, M.; Jensen-Urstad, M.; Ostergren, J.; Rosenqvist, M. Asymptomatic versus symptomatic persistent atrial fibrillation: Clinical and noninvasive characteristics. J. Intern. Med. 2001, 250, 390–397. [Google Scholar] [CrossRef] [PubMed]
- De Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.S.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.E.; Naditch-Brûlé, L.; Murin, J.; Goethals, M.; Inoue, H.; O’Neill, J.; Silva-Cardoso, J.; Zharinov, O.; Gamra, H.; Alam, S.; et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: Insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ. Arrhythmia Electrophysiol. 2012, 5, 632–639. [Google Scholar] [CrossRef]
- Gardarsdottir, M.; Sigurdsson, S.; Aspelund, T.; Rokita, H.; Launer, L.J.; Gudnason, V.; Arnar, D.O. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2018, 20, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Agarwal, S.K.; Norby, F.L.; Gottesman, R.F.; Loehr, L.R.; Soliman, E.Z.; Mosley, T.H.; Folsom, A.R.; Coresh, J.; Alonso, A. Persistent but not Paroxysmal Atrial Fibrillation Is Independently Associated With Lower Cognitive Function: ARIC Study. J. Am. Coll. Cardiol. 2016, 67, 1379–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chung, M.K.; Allen, L.A.; Ezekowitz, M.; Furie, K.L.; McCabe, P.; Noseworthy, P.A.; Perez, M.V.; Turakhia, M.P.; American Heart Association Council on Clinical, C.; et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e623–e644. [Google Scholar] [CrossRef] [PubMed]
| No AF (N = 7495) | AF (N = 974) | |
|---|---|---|
| Age, years, mean (SD) | 67.2 (9.2) | 72.4 (9.0) |
| Female, % | 35.8 | 29.4 |
| Education, % | ||
| Less than high school | 8.1 | 6.9 |
| High school and/or vocational school | 24.4 | 22.5 |
| At least some college | 67.6 | 70.6 |
| Race-ethnicity, % | ||
| Non-Hispanic White | 56.1 | 76.8 |
| Non-Hispanic Black | 31.2 | 15.9 |
| Hispanic | 11.0 | 5.3 |
| Other | 1.8 | 2.0 |
| BMI (kg/m2) | 29.8 (5.6) | 29.6 (5.4) |
| Systolic blood pressure (mmHg), mean (SD) | 139.6 (15.4) | 139.6 (16.2) |
| Diastolic blood pressure (mmHg), mean (SD) | 78.5 (11.7) | 74.9 (12.2) |
| History of CVD a, % | 14.7 | 36.3 |
| On oral anticoagulants, % | 1.2 | 20.9 |
| eGFR (mL/min per 1.73 m2), mean (SD) | 72.5 (20.4) | 67.3 (20.1) |
| HDL cholesterol (mg/dL), mean (SD) | 52.7 (14.4) | 53.0 (14.3) |
| Total cholesterol (mg/dL), mean (SD) | 191.5 (41.1) | 178.9 (39.5) |
| Currently smoking, % | 13.5 | 8.1 |
| CHA₂DS₂-VASc score b | 2.3 (1.1) | 2.9 (1.1) |
| ASCVD 10-year risk score c | 21% | 28% |
| MOCA score | 23.0 (4.0) | 23.1 (3.9) |
| Logical memory: immediate recall score | 19.3 (4.8) | 19.2 (4.9) |
| Logical memory: delayed recall score | 8.3 (3.3) | 8.1 (3.4) |
| Digit symbol coding score | 51.4 (15.3) | 49.5 (14.2) |
| Cognitive Outcomes | ||
| Probable dementia, % | 3.5 | 5.2 |
| Mild cognitive impairment, % | 7.4 | 8.5 |
| Combinations of Diagnostic Sources | Classification in Analysis | N | ||
|---|---|---|---|---|
| Self-Report | Safety Event | ECG | ||
| Self-report | 443 | |||
| Safety event | 149 | |||
| ECG | 97 | |||
| Safety event | 72 | |||
| ECG | 118 | |||
| ECG | 57 | |||
| ECG | 38 | |||
| TOTAL: | 974 | |||
| No AF (N = 7495) | AF (N = 974) | |
|---|---|---|
| Probable Dementia | ||
| N. cases | 265 | 51 |
| Person years | 36,093 | 4491 |
| Incidence rate * | 7.3 | 11.4 |
| Model 1 | 1 (Ref.) | 1.08 (0.78, 1.50) |
| Model 2 | 1 (Ref.) | 1.04 (0.73, 1.48) |
| MCI | ||
| N. cases | 551 | 83 |
| Person years | 34,410 | 4223 |
| Incidence rate | 16.0 | 19.7 |
| Model 1 | 1 (Ref.) | 1.06 (0.82, 1.38) |
| Model 2 | 1 (Ref.) | 1.14 (0.86, 1.50) |
| Probable dementia or MCI | ||
| N. cases | 738 | 120 |
| Person years | 34,716 | 4290 |
| Incidence rate | 21.3 | 28.0 |
| Model 1 | 1 (Ref.) | 0.96 (0.77, 1.19) |
| Model 2 | 1 (Ref.) | 0.96 (0.76, 1.22) |
| No AF (N = 7495) | Self-Report Only AF (N = 443) | Safety Event AF Only or Self-Report + Safety Events (N = 221) | ECG AF (N = 310) | |
|---|---|---|---|---|
| Probable Dementia | ||||
| N. cases | 265 | 18 | 12 | 21 |
| Person years | 36,093 | 2108 | 1001 | 1383 |
| Incidence rate * | 7.3 | 8.5 | 12.0 | 15.2 |
| Model 1 | 1 (Ref.) | 0.88 (0.58, 1.34) | 1.19 (0.68, 2.08) | 1.40 (0.79, 2.48) |
| Model 2 | 1 (Ref.) | 0.88 (0.57, 1.37) | 1.10 (0.63, 1.92) | 1.41 (0.78, 2.54) |
| MCI | ||||
| N. cases | 551 | 26 | 20 | 37 |
| Person years | 34,410 | 2007 | 939 | 1278 |
| Incidence rate | 16.0 | 13.0 | 21.3 | 29.0 |
| Model 1 | 1 (Ref.) | 0.85 (0.61, 1.19) | 1.03 (0.62, 1.71) | 1.52 (0.96, 2.40) |
| Model 2 | 1 (Ref.) | 0.91 (0.64, 1.30) | 1.04 (0.62, 1.74) | 1.53 (0.95, 2.47) |
| Prob dementia or MCI | ||||
| N. cases | 738 | 40 | 28 | 52 |
| Person years | 34,716 | 2035 | 953 | 1303 |
| Incidence rate | 21.3 | 19.7 | 29.4 | 39.9 |
| Model 1 | 1 (Ref.) | 0.74 (0.56, 0.97) | 0.97 (0.63, 1.48) | 1.60 (1.08, 2.37) |
| Model 2 | 1 (Ref.) | 0.75 (0.55, 1.01) | 0.94 (0.62, 1.45) | 1.59 (1.06, 2.38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.B.; Kulshreshtha, A.; Li, L.; Subramanya, V.; Alonso, A. Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials. J. Clin. Med. 2022, 11, 5800. https://doi.org/10.3390/jcm11195800
Alam AB, Kulshreshtha A, Li L, Subramanya V, Alonso A. Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials. Journal of Clinical Medicine. 2022; 11(19):5800. https://doi.org/10.3390/jcm11195800
Chicago/Turabian StyleAlam, Aniqa B., Ambar Kulshreshtha, Linzi Li, Vinita Subramanya, and Alvaro Alonso. 2022. "Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials" Journal of Clinical Medicine 11, no. 19: 5800. https://doi.org/10.3390/jcm11195800
APA StyleAlam, A. B., Kulshreshtha, A., Li, L., Subramanya, V., & Alonso, A. (2022). Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials. Journal of Clinical Medicine, 11(19), 5800. https://doi.org/10.3390/jcm11195800






