Inflammatory Surrogate Parameters for Predicting Ifosfamide-Induced Neurotoxicity in Sarcoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Data
2.3. Data Analysis
3. Results
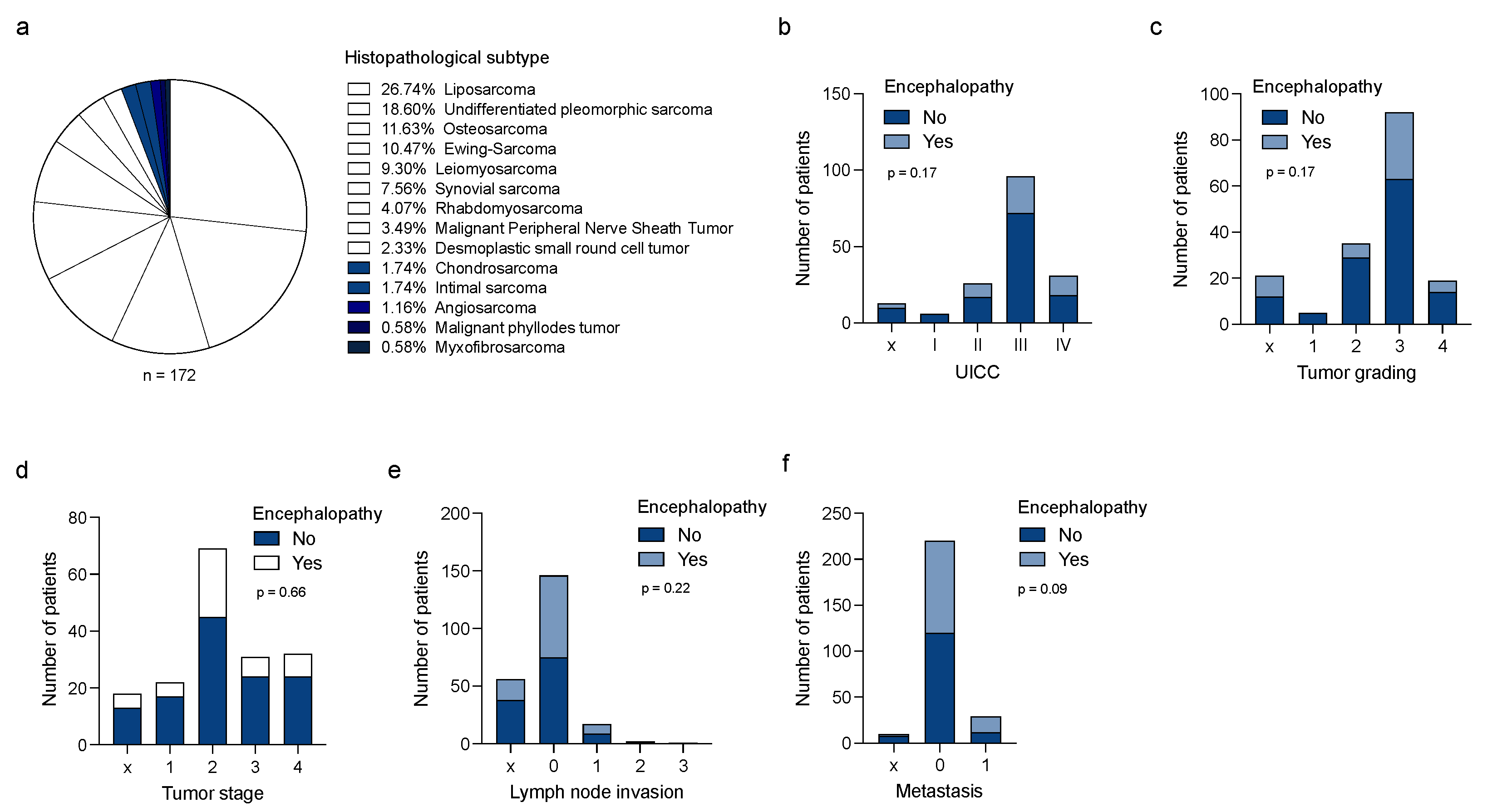
3.1. Ifosfamide-Induced Neurotoxicity (IIN) Is Independent of Tumor Entity or Tumor Stages
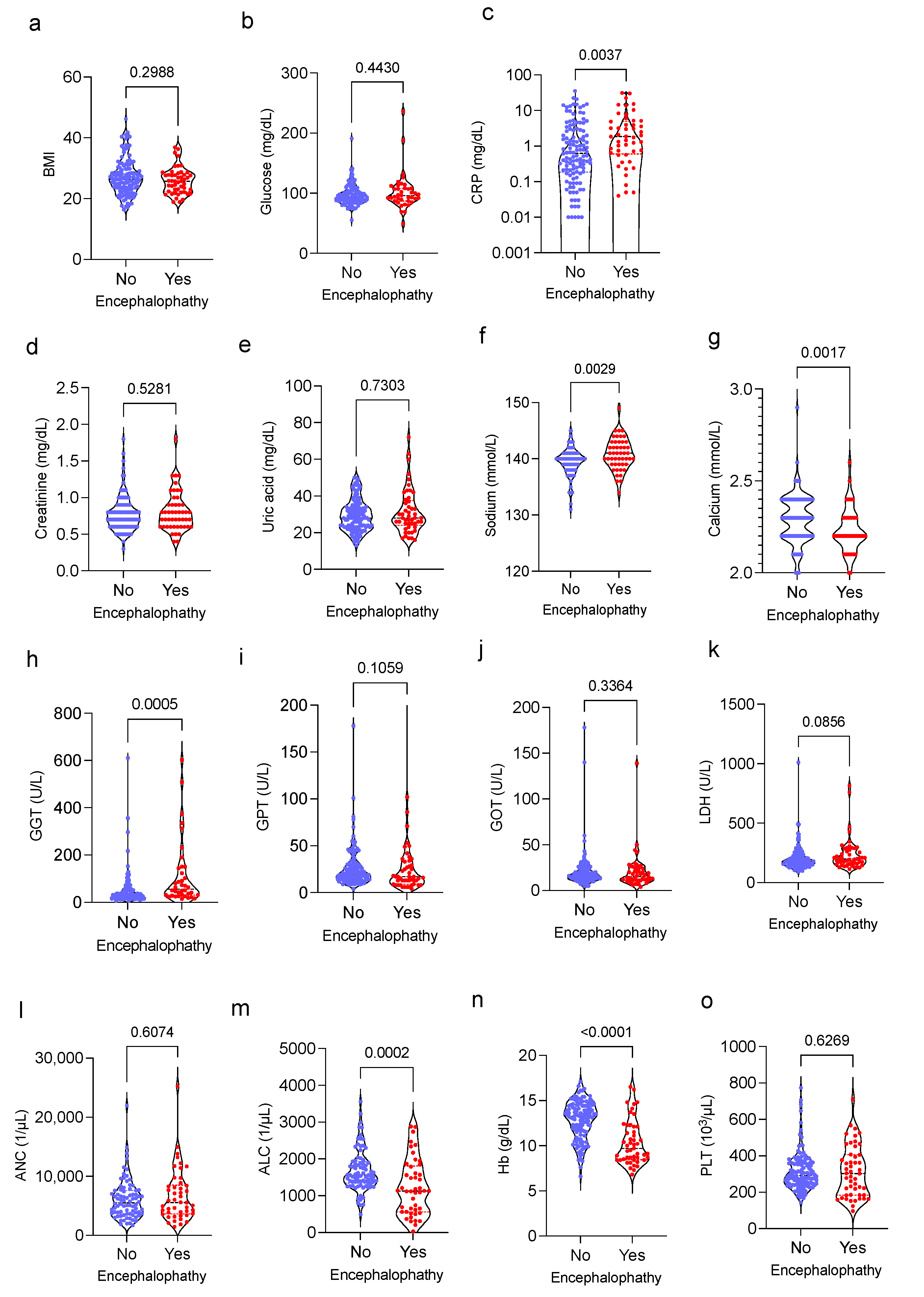
3.2. Association of Routinely Determined Laboratory Markers and IIN
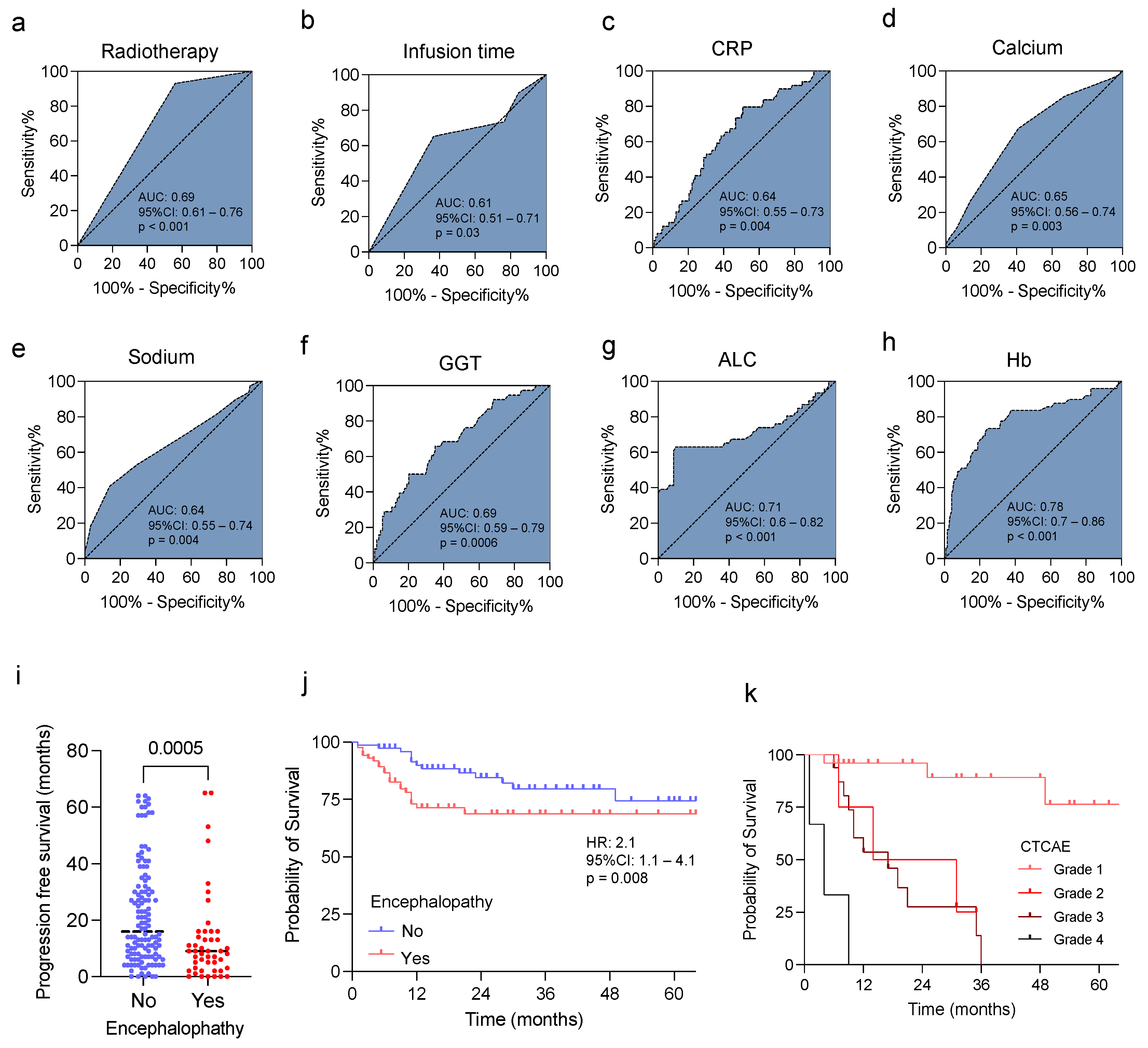
3.3. Predictive Value of Clinical and Laboratory Findings and IIN in Sarcoma Patients
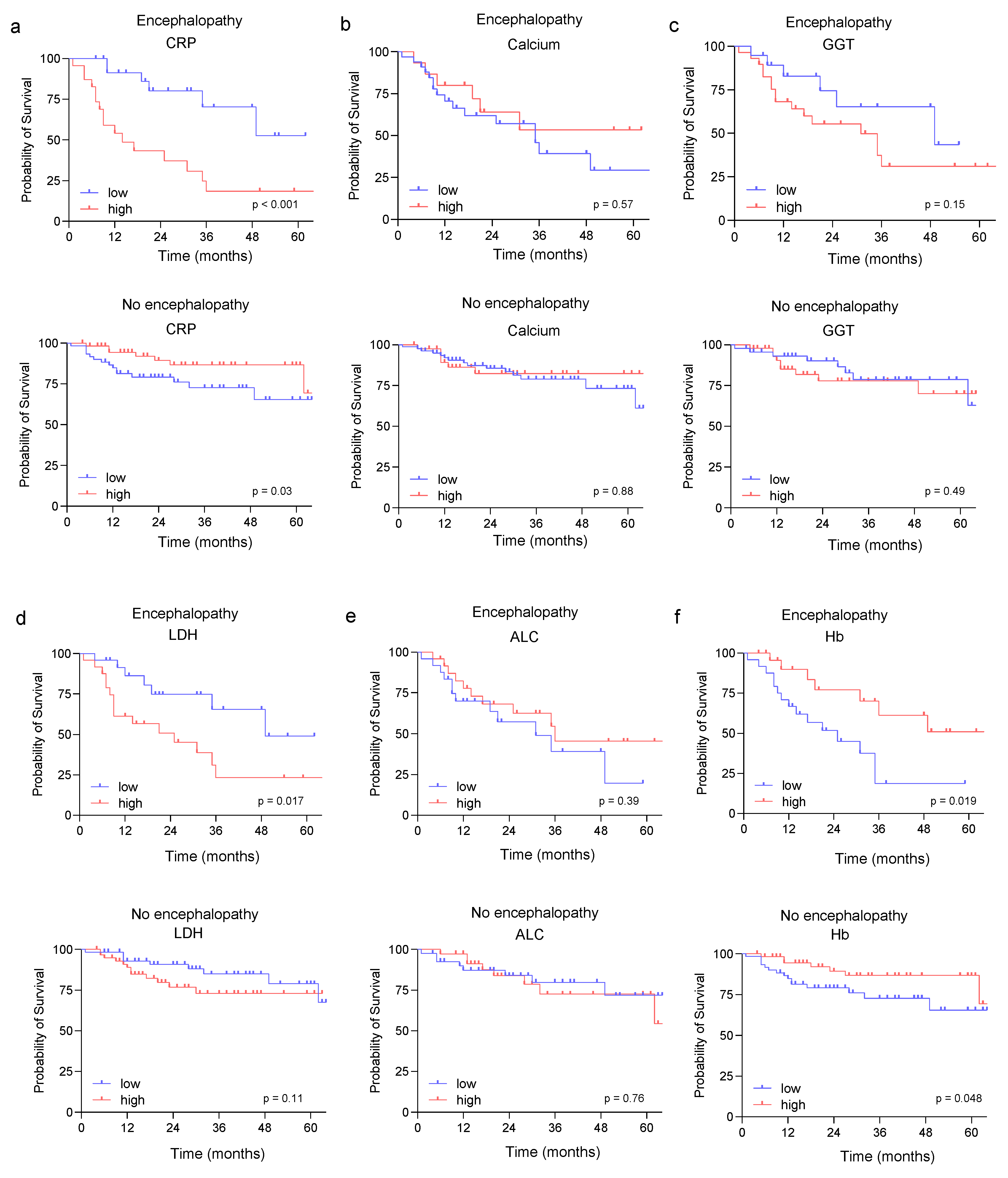
3.4. Prognostic Role of the Identified Risk Factors in Sarcoma Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nacev, B.A.; Sanchez-Vega, F.; Smith, S.A.; Antonescu, C.R.; Rosenbaum, E.; Shi, H.; Tang, C.; Socci, N.D.; Rana, S.; Gularte-Mérida, R.; et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nat. Commun. 2022, 13, 3405. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.T.; Acevedo, L.A.; Mann, M.J.; Tolani, B. A review of soft-tissue sarcomas: Translation of biological advances into treatment measures. Cancer Manag. Res. 2018, 10, 1089–1114. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.; Tsagozis, P. Multidisciplinary treatment of soft tissue sarcomas: An update. World J. Clin. Oncol. 2020, 11, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Nakano, K. Challenges of Systemic Therapy Investigations for Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 3540. [Google Scholar] [CrossRef]
- Bläsius, F.; Delbrück, H.; Hildebrand, F.; Hofmann, U.K. Surgical Treatment of Bone Sarcoma. Cancers 2022, 14, 2694. [Google Scholar] [CrossRef]
- Demetri, G.D.; Elias, A.D. Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol. Oncol. Clin. N. Am. 1995, 9, 765–785. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- García-Del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022, 13, 158–163. [Google Scholar] [CrossRef]
- Schöffski, P. Established and experimental systemic treatment options for advanced liposarcoma. Oncol. Res. Treat 2022, 45, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.P.; Doroshow, D.B.; Do, K.T.; Keedy, V.L.; Sklar, J.S.; Glazer, P.; Bindra, R.; Shapiro, G.I. Clinical Efficacy of Olaparib in IDH1/IDH2-Mutant Mesenchymal Sarcomas. JCO Precis. Oncol. 2021, 5, 466–472. [Google Scholar] [CrossRef]
- Klastersky, J. Side effects of ifosfamide. Oncology 2003, 65 (Suppl. S2), 7–10. [Google Scholar] [CrossRef]
- Verstappen, C.C.; Heimans, J.J.; Hoekman, K.; Postma, T.J. Neurotoxic complications of chemotherapy in patients with cancer: Clinical signs and optimal management. Drugs 2003, 63, 1549–1563. [Google Scholar] [CrossRef]
- Savica, R.; Rabinstein, A.A.; Josephs, K.A. Ifosfamide associated myoclonus-encephalopathy syndrome. J. Neurol. 2011, 258, 1729–1731. [Google Scholar] [CrossRef]
- Tajino, T.; Kikuchi, S.; Yamada, H.; Takeda, A.; Konno, S. Ifosfamide encephalopathy associated with chemotherapy for musculoskeletal sarcomas: Incidence, severity, and risk factors. J. Orthop. Sci. 2010, 15, 104–111. [Google Scholar] [CrossRef]
- Janeway, K.A.; Grier, H.E. Sequelae of osteosarcoma medical therapy: A review of rare acute toxicities and late effects. Lancet Oncol. 2010, 11, 670–678. [Google Scholar] [CrossRef]
- Sweiss, K.I.; Beri, R.; Shord, S.S. Encephalopathy after high-dose Ifosfamide: A retrospective cohort study and review of the literature. Drug Saf. 2008, 31, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Rieger, C.; Fiegl, M.; Tischer, J.; Ostermann, H.; Schiel, X. Incidence and severity of ifosfamide-induced encephalopathy. Anticancer Drugs 2004, 15, 347–350. [Google Scholar] [CrossRef] [PubMed]
- DiMaggio, J.R.; Brown, R.; Baile, W.F.; Schapira, D. Hallucinations and ifosfamide-induced neurotoxicity. Cancer 1994, 73, 1509–1514. [Google Scholar] [CrossRef]
- Merimsky, O.; Reider-Groswasser, I.; Wigler, N.; Chaitchik, S. Encephalopathy in ifosfamide-treated patients. Acta Neurol. Scand. 1992, 86, 521–525. [Google Scholar] [CrossRef]
- Curtin, J.P.; Koonings, P.P.; Gutierrez, M.; Schlaerth, J.B.; Morrow, C.P. Ifosfamide-induced neurotoxicity. Gynecol. Oncol. 1991, 42, 193–196; discussion 191–192. [Google Scholar] [CrossRef]
- Turner, A.R.; Duong, C.D.; Good, D.J. Methylene blue for the treatment and prophylaxis of ifosfamide-induced encephalopathy. Clin. Oncol. 2003, 15, 435–439. [Google Scholar] [CrossRef]
- Ajithkumar, T.; Parkinson, C.; Shamshad, F.; Murray, P. Ifosfamide encephalopathy. Clin. Oncol. 2007, 19, 108–114. [Google Scholar] [CrossRef]
- Nicolao, P.; Giometto, B. Neurological toxicity of ifosfamide. Oncology 2003, 65 (Suppl. S2), 11–16. [Google Scholar] [CrossRef]
- Lokiec, F. Ifosfamide: Pharmacokinetic properties for central nervous system metastasis prevention. Ann Oncol 2006, 17 (Suppl. S4), iv33–iv36. [Google Scholar] [CrossRef]
- Richards, A.; Marshall, H.; McQuary, A. Evaluation of methylene blue, thiamine, and/or albumin in the prevention of ifosfamide-related neurotoxicity. J. Oncol. Pharm. Pract. 2011, 17, 372–380. [Google Scholar] [CrossRef]
- Pelgrims, J.; De Vos, F.; Van den Brande, J.; Schrijvers, D.; Prove, A.; Vermorken, J.B. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: Report of 12 cases and a review of the literature. Br. J. Cancer 2000, 82, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Zustovich, F.; Nicoletto, M.O.; Donach, M.; Pastorelli, D. Important role of thiamine in preventing ifosfamide-induced encephalopathy. J. Oncol. Pharm. Pract. 2010, 16, 135–136. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, H.J.; Lee, Y.S.; Hwang, J.S.; Lee, M.S. Ifosfamide-induced encephalopathy with or without using methylene blue. Int. J. Gynecol. Cancer 2005, 15, 807–810. [Google Scholar] [CrossRef]
- Szabatura, A.H.; Cirrone, F.; Harris, C.; McDonnell, A.M.; Feng, Y.; Voit, D.; Neuberg, D.; Butrynski, J.; Fisher, D.C. An assessment of risk factors associated with ifosfamide-induced encephalopathy in a large academic cancer center. J. Oncol. Pharm. Pract. 2015, 21, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ouali, M.; van Glabbeke, M.; Krarup-Hansen, A.; Rodenhuis, S.; Le Cesne, A.; Hogendoorn, P.C.; Verweij, J.; Blay, J.Y. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur. J. Cancer 2010, 46, 72–83. [Google Scholar] [CrossRef]
- Nakamura, T.; Katagiri, H.; Shido, Y.; Hamada, S.; Yamada, K.; Nagano, A.; Yamada, S.; Tsukushi, S.; Ishimura, D.; Matsumine, A.; et al. Analysis of Factors for Predicting Survival in Soft-tissue Sarcoma with Metastatic Disease at Initial Presentation. Anticancer Res. 2017, 37, 3137–3141. [Google Scholar] [CrossRef][Green Version]
- Howell, J.E.; Szabatura, A.H.; Seung, A.H.; Nesbit, S.A. Characterization of the occurrence of ifosfamide-induced neurotoxicity with concomitant aprepitant. J. Oncol. Pharm. Pract. 2008, 14, 157–162. [Google Scholar] [CrossRef]
- David, K.A.; Picus, J. Evaluating risk factors for the development of ifosfamide encephalopathy. Am. J. Clin. Oncol. 2005, 28, 277–280. [Google Scholar] [CrossRef]
- Goren, M.P.; Wright, R.K.; Pratt, C.B.; Horowitz, M.E.; Dodge, R.K.; Viar, M.J.; Kovnar, E.H. Potentiation of ifosfamide neurotoxicity, hematotoxicity, and tubular nephrotoxicity by prior cis-diamminedichloroplatinum(II) therapy. Cancer Res. 1987, 47, 1457–1460. [Google Scholar]
- Lo, Y.; Shen, L.J.; Chen, W.H.; Dong, Y.H.; Wu, F.L. Risk factors of ifosfamide-related encephalopathy in adult patients with cancer: A retrospective analysis. J. Formos. Med. Assoc. 2016, 115, 744–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antman, K.H.; Elias, A.; Ryan, L. Ifosfamide and mesna: Response and toxicity at standard- and high-dose schedules. Semin. Oncol. 1990, 17, 68–73. [Google Scholar]
- Perren, T.J.; Turner, R.C.; Smith, I.E. Encephalopathy with rapid infusion ifosfamide/mesna. Lancet 1987, 329, 390–391. [Google Scholar] [CrossRef]
- Salloum, E.; Flamant, F.; Ghosn, M.; Taleb, N.; Akatchereian, C. Irreversible encephalopathy with ifosfamide/mesna. J. Clin. Oncol. 1987, 5, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Kettle, J.K.; Grauer, D.; Folker, T.L.; O’Neal, N.; Henry, D.W.; Williams, C.B. Effectiveness of exogenous albumin administration for the prevention of ifosfamide-induced encephalopathy. Pharmacotherapy 2010, 30, 812–817. [Google Scholar] [CrossRef]
- Meanwell, C.A.; Blake, A.E.; Kelly, K.A.; Honigsberger, L.; Blackledge, G. Prediction of ifosfamide/mesna associated encephalopathy. Eur. J. Cancer Clin. Oncol. 1986, 22, 815–819. [Google Scholar] [CrossRef]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef]
- Klein, G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Szkandera, J.; Absenger, G.; Liegl-Atzwanger, B.; Pichler, M.; Stotz, M.; Samonigg, H.; Glehr, M.; Zacherl, M.; Stojakovic, T.; Gerger, A.; et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br. J. Cancer 2013, 108, 1677–1683. [Google Scholar] [CrossRef]
- Brewster, R.; Purington, N.; Henry, S.; Wood, D.; Ganjoo, K.; Bui, N. Evaluation of Absolute Lymphocyte Count at Diagnosis and Mortality Among Patients With Localized Bone or Soft Tissue Sarcoma. JAMA Netw. Open 2021, 4, e210845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ma, S.; Hua, Z.; Zhao, Z.; Gao, S. Prognostic Value of Pretreated Blood Inflammatory Markers in Patients with Bone Sarcoma: A Meta-Analysis. Dis. Markers 2021, 2021, 8839512. [Google Scholar] [CrossRef] [PubMed]
- Danielski, L.G.; Giustina, A.D.; Badawy, M.; Barichello, T.; Quevedo, J.; Dal-Pizzol, F.; Petronilho, F. Brain Barrier Breakdown as a Cause and Consequence of Neuroinflammation in Sepsis. Mol. Neurobiol. 2018, 55, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Total | (%) |
|---|---|---|
| (n = 172) | ||
| Gender | ||
| female, sex (n) | 63 | 36.6 |
| Age | 18 to 76 | |
| Age in years, mean–yr. ± SD | 60.0 +/− 14.9 | |
| TNM classification | ||
| Stage | ||
| Tx | 18 | 10.4 |
| T1 | 22 | 12.8 |
| T2 | 69 | 40.1 |
| T3 | 31 | 18.1 |
| T4 | 32 | 18.6 |
| Node | ||
| Nx | 56 | 32.6 |
| N0 | 96 | 55.8 |
| N1 | 17 | 9.8 |
| N2 | 2 | 1.2 |
| N3 | 1 | 0.6 |
| Metastasis | ||
| Mx | 10 | 5.8 |
| M0 | 132 | 76.7 |
| M1 | 30 | 17.5 |
| Histological grading, n (%) | ||
| Gx | 21 | 12.2 |
| G1 | 5 | 2.9 |
| G2 | 35 | 20.3 |
| G3 | 92 | 53.5 |
| G4 | 19 | 11.1 |
| Histology | ||
| Angiosarcoma | 2 | 1.2 |
| Chondrosarcoma | 3 | 1.7 |
| Desmoplastic small round cell tumor | 4 | 2.3 |
| Ewing Sarcoma | 18 | 10.5 |
| Intimal sarcoma | 3 | 1.7 |
| Leiomyosarcoma | 16 | 9.3 |
| Liposarcoma | 46 | 26.7 |
| Malignant Peripheral Nerve Sheath Tumor | 6 | 3.5 |
| Malignant phylloides tumor | 1 | 0.6 |
| Myxofibrosarcoma | 1 | 0.6 |
| Osteosarcoma | 20 | 11.6 |
| Rhabdomyosarcoma | 7 | 4.1 |
| Synovial sarcoma | 13 | 7.6 |
| Undifferentiated pleomorphic sarcoma | 32 | 18.6 |
| UICC stage | ||
| X | 13 | 7.6 |
| I | 6 | 3.5 |
| II | 26 | 15.1 |
| III | 96 | 55.8 |
| IV | 31 | 18.0 |
| Therapy line at time-point of treatment with ifosfamide | ||
| 1st line | 166 | 96.5 |
| 2nd line | 5 | 2.9 |
| >2nd line | 1 | 0.6 |
| Treatment setting | ||
| Neoadjuvant | 107 | 62.2 |
| Adjuvant | 43 | 25.0 |
| Neoadjuvant + adjuvant | 16 | 9.3 |
| Palliative (primarily) | 25 | 14.5 |
| Combined radiochemotherapy | 49 | 28.5 |
| Surgery | 154 | 89.5 |
| Concurrent therapy | ||
| Monotherapy | 52 | 30.2 |
| Anthracycline-based | 77 | 44.8 |
| Platinum- and Anthracycline-based | 15 | 8.7 |
| 1 combinatorial treatment partner | 78 | 45.3 |
| 2 combinatorial treatment partners | 19 | 11.0 |
| >2 combinatorial treatment partners | 23 | 13.4 |
| Ifosfamide daily dose (mg/m²/day) | ||
| 3000 | 172 | 100.0 |
| Number of therapy cycles | ||
| 1–3 | 95 | 55.2 |
| 4–6 | 67 | 39.0 |
| >6 | 10 | 5.8 |
| Infusion time | ||
| 1 h | 23 | 13.4 |
| 2 h | 15 | 8.7 |
| 3 h | 4 | 2.3 |
| 4 h | 53 | 30.8 |
| 24 h | 77 | 44.8 |
| Comorbidities | ||
| hypertension | 52 | 30.2 |
| hypothyroidism | 15 | 8.7 |
| diabetes mellitus | 13 | 7.6 |
| chronic kidney disease | 8 | 4.7 |
| bronchial asthma | 7 | 4.1 |
| Encephalopathy | No (n = 123) | Yes (n = 49) | p-Value |
|---|---|---|---|
| Treatment | |||
| concurrent doxorubicin | 47 (38.2%) | 30 (61.2%) | 0.06 |
| concurrent cisplatin | 10 (8.1%) | 5 (10.2%) | 0.67 |
| concurrent vincristine | 19 (15.4%) | 7 (14.3%) | 0.85 |
| concurrent etoposide | 18 (14.6%) | 6 (12.2%) | 0.68 |
| Infusion time | |||
| 1 h | 18 (14.6%) | 5 (10.2%) | 0.007 |
| 2 h | 9 (7.3%) | 6 (12.2%) | |
| 3 h | 2 (1.6%) | 2 (4.1%) | |
| 4 h | 47 (38.2%) | 6 (12.2%) | |
| 24 h | 47 (38.2%) | 30 (61.2%) | |
| Mean | 11.04 | 15.65 | 0.009 |
| SD | 10.28 | 10.62 | |
| Cycle | |||
| 1st | x | 35 (71.4%) | |
| 2nd and 3rd | x | 6 (12.3%) | |
| >3rd | x | 8 (16.3%) | |
| Radiotherapy | |||
| Yes | 44 (35.8%) | 5 (10.2%) | 0.0008 |
| No | 79 (64.2%) | 44 (89.8%) | |
| Co-medication | |||
| opioids | 21 (17.1%) | 20 (40.8%) | 0.85 |
| anticonvulsant | 11 (8.9%) | 13 (26.5%) | |
| antidepressant | 9 (7.3%) | 7 (14.3%) | |
| anxiolytic | 1 (0.8%) | 1 (2.0%) | |
| neuroleptic | 1 (0.8%) | 0 (0%) | |
| Age | 49.9 +/− 14.09 | 53.61 +/− 16.51 | 0.14 |
| Sex | |||
| Male | 81 (65.9%) | 28 (57.1%) | 0.284 |
| Female | 42 (34.1%) | 21 (42.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, M.; Benzler, K.; Lauer, U.M.; Zender, L.; Hinterleitner, C.; Hinterleitner, M. Inflammatory Surrogate Parameters for Predicting Ifosfamide-Induced Neurotoxicity in Sarcoma Patients. J. Clin. Med. 2022, 11, 5798. https://doi.org/10.3390/jcm11195798
Schmidt M, Benzler K, Lauer UM, Zender L, Hinterleitner C, Hinterleitner M. Inflammatory Surrogate Parameters for Predicting Ifosfamide-Induced Neurotoxicity in Sarcoma Patients. Journal of Clinical Medicine. 2022; 11(19):5798. https://doi.org/10.3390/jcm11195798
Chicago/Turabian StyleSchmidt, Moritz, Katrin Benzler, Ulrich M. Lauer, Lars Zender, Clemens Hinterleitner, and Martina Hinterleitner. 2022. "Inflammatory Surrogate Parameters for Predicting Ifosfamide-Induced Neurotoxicity in Sarcoma Patients" Journal of Clinical Medicine 11, no. 19: 5798. https://doi.org/10.3390/jcm11195798
APA StyleSchmidt, M., Benzler, K., Lauer, U. M., Zender, L., Hinterleitner, C., & Hinterleitner, M. (2022). Inflammatory Surrogate Parameters for Predicting Ifosfamide-Induced Neurotoxicity in Sarcoma Patients. Journal of Clinical Medicine, 11(19), 5798. https://doi.org/10.3390/jcm11195798




