Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ethics
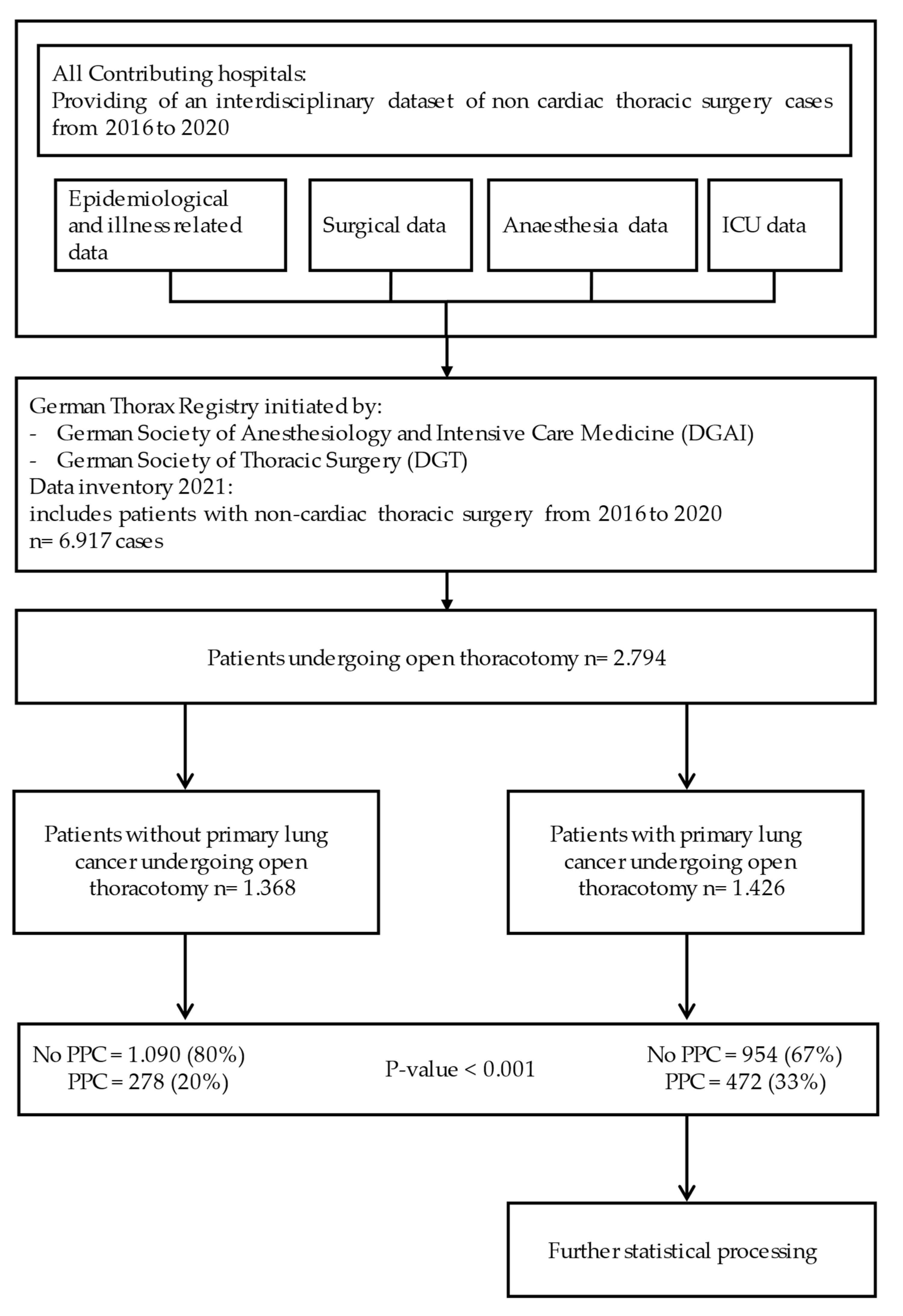
2.3. Patients
2.4. Parameters
2.5. Statistics
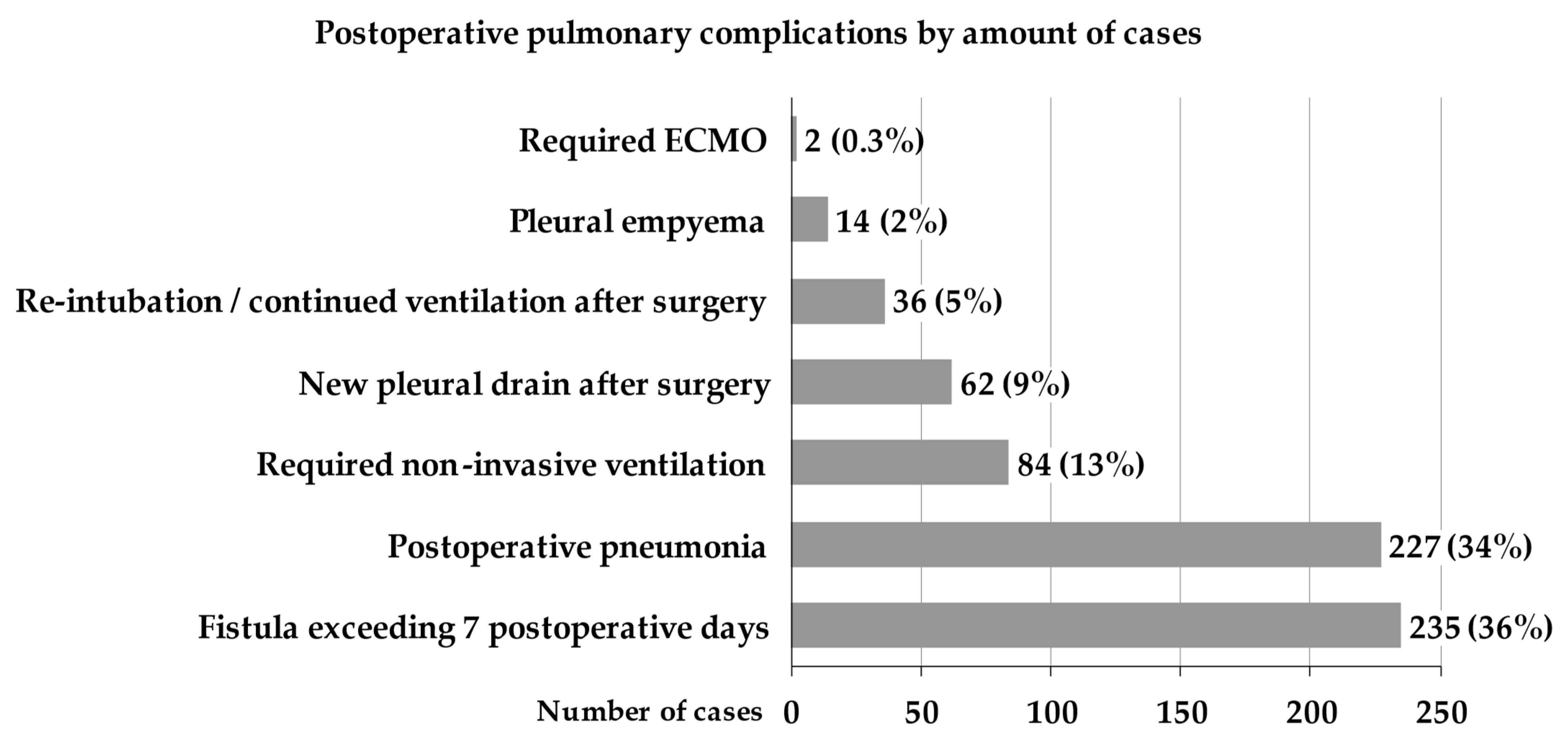
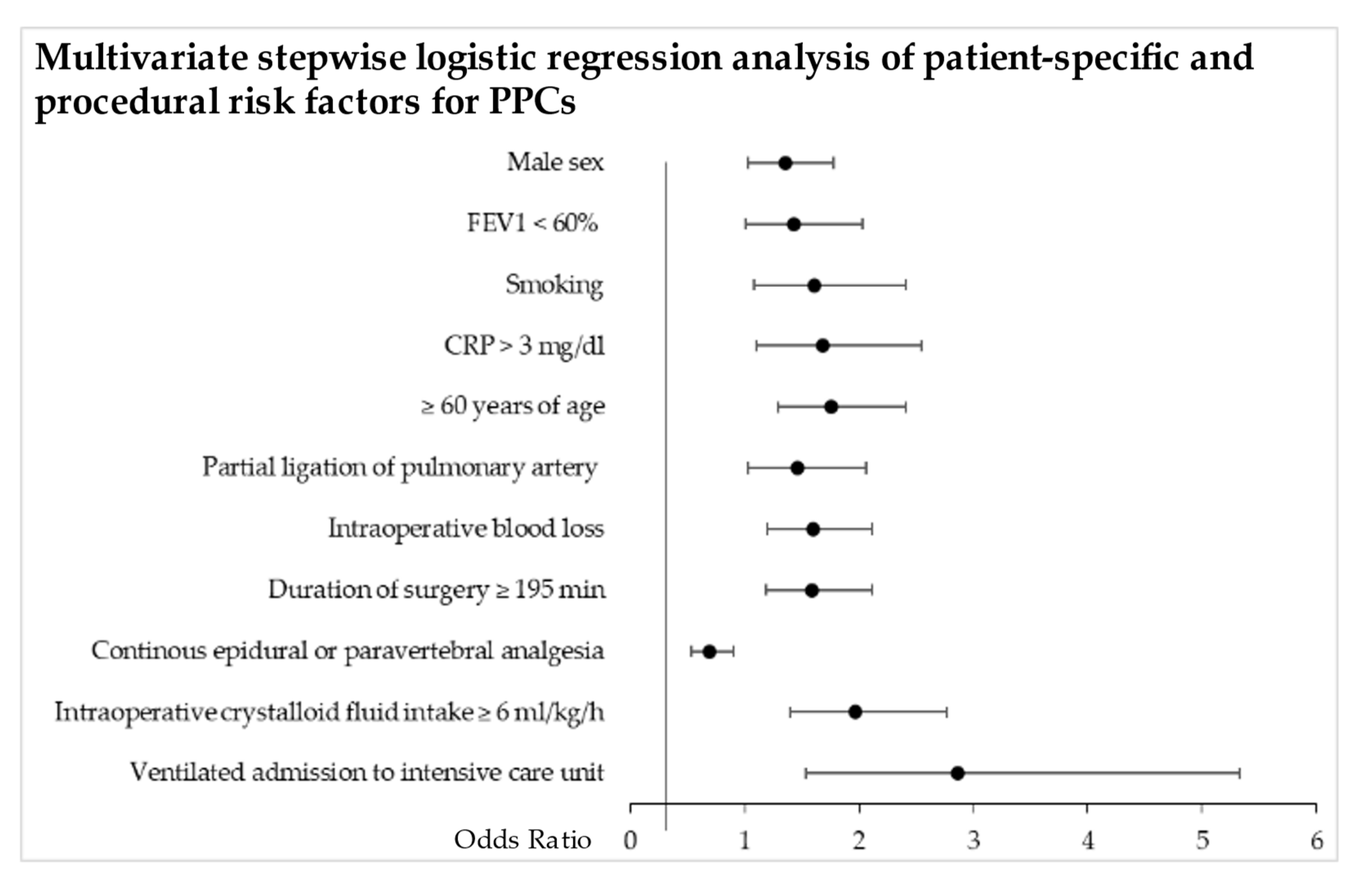
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jani, C.; Marshall, D.C.; Singh, H.; Goodall, R.; Shalhoub, J.; Al Omari, O.; Salciccioli, J.D.; Thomson, C.C. Lung Cancer Mortality in Europe and the USA between 2000 and 2017: An Observational Analysis. ERJ Open Res. 2021, 7, 00311–02021. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K.; American College of Chest Physicians. Treatment of Non-Small Cell Lung Cancer Stage I and Stage II: ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition). Chest 2007, 132, 234S–242S. [Google Scholar] [CrossRef] [PubMed]
- Shelley, B.G.; McCall, P.J.; Glass, A.; Orzechowska, I.; Klein, A.A.; Association of Cardiothoracic Anaesthesia and collaborators. Association between Anaesthetic Technique and Unplanned Admission to Intensive Care after Thoracic Lung Resection Surgery: The Second Association of Cardiothoracic Anaesthesia and Critical Care (ACTACC) National Audit. Anaesthesia 2019, 74, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Bhamidipati, C.M.; Lau, C.L.; Jones, D.R.; Kozower, B.D. The Society of Thoracic Surgeons General Thoracic Surgery Database: Establishing Generalizability to National Lung Cancer Resection Outcomes. Ann. Thorac. Surg. 2012, 94, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Pilling, J.E.; Martin-Ucar, A.E.; Waller, D.A. Salvage Intensive Care Following Initial Recovery from Pulmonary Resection: Is It Justified? Ann. Thorac. Surg. 2004, 77, 1039–1044. [Google Scholar] [CrossRef]
- Sen, S.; Sen, S.; Sentürk, E.; Kuman, N.K. Postresectional Lung Injury in Thoracic Surgery Pre and Intraoperative Risk Factors: A Retrospective Clinical Study of a Hundred Forty-Three Cases. J. Cardiothorac. Surg. 2010, 5, 62. [Google Scholar] [CrossRef]
- Agostini, P.J.; Lugg, S.T.; Adams, K.; Smith, T.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B.; Rushton, A.; Bishay, E. Risk Factors and Short-Term Outcomes of Postoperative Pulmonary Complications after VATS Lobectomy. J. Cardiothorac. Surg. 2018, 13, 28. [Google Scholar] [CrossRef]
- Traibi, A.; Grigoroiu, M.; Boulitrop, C.; Urena, A.; Masuet-Aumatell, C.; Brian, E.; Stern, J.-B.; Zaimi, R.; Gossot, D. Predictive Factors for Complications of Anatomical Pulmonary Segmentectomies. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 838–844. [Google Scholar] [CrossRef]
- Arslantas, M.K.; Kara, H.V.; Tuncer, B.B.; Yildizeli, B.; Yuksel, M.; Bostanci, K.; Bekiroglu, N.; Kararmaz, A.; Cinel, I.; Batirel, H.F. Effect of the Amount of Intraoperative Fluid Administration on Postoperative Pulmonary Complications Following Anatomic Lung Resections. J. Thorac. Cardiovasc. Surg. 2015, 149, 314–321.e1. [Google Scholar] [CrossRef]
- Kaufmann, K.B.; Loop, T.; Heinrich, S.; Working Group of the German Thorax Registry. Risk Factors for Post-Operative Pulmonary Complications in Lung Cancer Patients after Video-Assisted Thoracoscopic Lung Resection: Results of the German Thorax Registry. Acta Anaesthesiol. Scand. 2019, 63, 1009–1018. [Google Scholar] [CrossRef]
- Defosse, J.; Schieren, M.; Loop, T.; Arndt, C.; Röhrig, R.; Stoelben, E.; Ludwig, C.; Schleppers, A.; Wappler, F.; Gerbershagen, M.; et al. The German Thorax Registry: Implementation of an Established Tool of Perioperative Health Care Research. Zentralbl. Chir. 2017, 142, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for Definitions and Use of Outcome Measures for Clinical Effectiveness Research in Perioperative Medicine: European Perioperative Clinical Outcome (EPCO) Definitions: A Statement from the ESA-ESICM Joint Taskforce on Perioperative Outcome Measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef]
- Agostini, P.; Cieslik, H.; Rathinam, S.; Bishay, E.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Singh, S.; Naidu, B. Postoperative Pulmonary Complications Following Thoracic Surgery: Are There Any Modifiable Risk Factors? Thorax 2010, 65, 815–818. [Google Scholar] [CrossRef]
- Alam, N.; Park, B.J.; Wilton, A.; Seshan, V.E.; Bains, M.S.; Downey, R.J.; Flores, R.M.; Rizk, N.; Rusch, V.W.; Amar, D. Incidence and Risk Factors for Lung Injury after Lung Cancer Resection. Ann. Thorac. Surg. 2007, 84, 1085–1091. [Google Scholar] [CrossRef]
- Ellenberger, C.; Garofano, N.; Reynaud, T.; Triponez, F.; Diaper, J.; Bridevaux, P.-O.; Karenovics, W.; Licker, M. Patient and Procedural Features Predicting Early and Mid-Term Outcome after Radical Surgery for Non-Small Cell Lung Cancer. J. Thorac. Dis. 2018, 10, 6020–6029. [Google Scholar] [CrossRef]
- Musallam, K.M.; Rosendaal, F.R.; Zaatari, G.; Soweid, A.; Hoballah, J.J.; Sfeir, P.M.; Zeineldine, S.; Tamim, H.M.; Richards, T.; Spahn, D.R.; et al. Smoking and the Risk of Mortality and Vascular and Respiratory Events in Patients Undergoing Major Surgery. JAMA Surg. 2013, 148, 755–762. [Google Scholar] [CrossRef]
- Licker, M.; de Perrot, M.; Spiliopoulos, A.; Robert, J.; Diaper, J.; Chevalley, C.; Tschopp, J.-M. Risk Factors for Acute Lung Injury after Thoracic Surgery for Lung Cancer. Anesth. Analg. 2003, 97, 1558–1565. [Google Scholar] [CrossRef]
- Chau, E.H.L.; Slinger, P. Perioperative Fluid Management for Pulmonary Resection Surgery and Esophagectomy. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 36–44. [Google Scholar] [CrossRef]
- Kaufmann, K.; Heinrich, S. Minimizing Postoperative Pulmonary Complications in Thoracic Surgery Patients. Curr. Opin. Anaesthesiol. 2021, 34, 13–19. [Google Scholar] [CrossRef]
- Loop, T. Fast Track in Thoracic Surgery and Anaesthesia: Update of Concepts. Curr. Opin. Anaesthesiol. 2016, 29, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kozian, A.; Schilling, T.; Hachenberg, T. Non-Analgetic Effects of Thoracic Epidural Anaesthesia. Curr. Opin. Anaesthesiol. 2005, 18, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Muehling, B.M.; Halter, G.L.; Schelzig, H.; Meierhenrich, R.; Steffen, P.; Sunder-Plassmann, L.; Orend, K.-H. Reduction of Postoperative Pulmonary Complications after Lung Surgery Using a Fast Track Clinical Pathway. Eur. J. Cardiothorac. Surg. 2008, 34, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for Enhanced Recovery after Lung Surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.Y.; Gates, S.; Naidu, B.V.; Wilson, M.J.A.; Gao Smith, F. Paravertebral Block versus Thoracic Epidural for Patients Undergoing Thoracotomy. Cochrane Database Syst. Rev. 2016, 2, CD009121. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Bonnet, F.; Shah, R.; Wilkinson, R.C.; Camu, F.; Fischer, B.; Neugebauer, E.A.M.; Rawal, N.; Schug, S.A.; Simanski, C.; et al. A Systematic Review of Randomized Trials Evaluating Regional Techniques for Postthoracotomy Analgesia. Anesth. Analg. 2008, 107, 1026–1040. [Google Scholar] [CrossRef]
- Ferrando, C.; Mugarra, A.; Gutierrez, A.; Carbonell, J.A.; García, M.; Soro, M.; Tusman, G.; Belda, F.J. Setting Individualized Positive End-Expiratory Pressure Level with a Positive End-Expiratory Pressure Decrement Trial after a Recruitment Maneuver Improves Oxygenation and Lung Mechanics during One-Lung Ventilation. Anesth. Analg. 2014, 118, 657–665. [Google Scholar] [CrossRef]
- Kiss, T.; Wittenstein, J.; Becker, C.; Birr, K.; Cinnella, G.; Cohen, E.; El Tahan, M.R.; Falcão, L.F.; Gregoretti, C.; Granell, M.; et al. Protective Ventilation with High versus Low Positive End-Expiratory Pressure during One-Lung Ventilation for Thoracic Surgery (PROTHOR): Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 213. [Google Scholar] [CrossRef]
- Im, Y.; Park, H.Y.; Shin, S.; Shin, S.H.; Lee, H.; Ahn, J.H.; Sohn, I.; Cho, J.H.; Kim, H.K.; Zo, J.I.; et al. Prevalence of and Risk Factors for Pulmonary Complications after Curative Resection in Otherwise Healthy Elderly Patients with Early Stage Lung Cancer. Respir. Res. 2019, 20, 136. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Li, Y.; Li, J.; Jiang, G.; Liu, J.; Wang, J. The Long-Term Impact of Postoperative Pulmonary Complications after Video-Assisted Thoracic Surgery Lobectomy for Lung Cancer. J. Thorac. Dis. 2017, 9, 5143–5152. [Google Scholar] [CrossRef]
- Seely, A.J.E.; Ivanovic, J.; Threader, J.; Al-Hussaini, A.; Al-Shehab, D.; Ramsay, T.; Gilbert, S.; Maziak, D.E.; Shamji, F.M.; Sundaresan, R.S. Systematic Classification of Morbidity and Mortality after Thoracic Surgery. Ann. Thorac. Surg. 2010, 90, 936–942. [Google Scholar] [CrossRef] [PubMed]

| No PPC (n = 954) | PPC (n = 472) | p-Value | |
|---|---|---|---|
| Patient-specific characteristics | |||
| Male gender | 541 (57%) | 315 (67%) | <0.001 |
| Age [years] | 65 ± 10 | 66 ± 9 | 0.001 |
| ≥60 years of age | 678 (71%) | 373 (79%) | 0.001 |
| BMI (mean +/−sd) | 26.5 ± 5.8 | 26.4 ± 6.2 | 0.675 |
| ≤19 | 38 (4%) | 22 (5%) | 0.549 |
| >30 | 201 (21%) | 100 (21%) | 0.959 |
| Smoking | |||
| never | 165 (17%) | 47 (10%) | <0.001 |
| current | 374 (39%) | 209 (44%) | 0.067 |
| cessation ≥ 3 months | 390 (41%) | 206 (44%) | 0.319 |
| ASA ≥ 3 | 900 (94%) | 431 (91%) | 0.031 |
| Neoadjuvant therapy | |||
| Radiation | 110 (12%) | 71 (15%) | 0.061 |
| Chemotherapy | 103 (11%) | 47 (10%) | 0.627 |
| Pre-operative characteristics | |||
| Re-thoracotomy | 73 (8%) | 45 (10%) | 0.225 |
| Pulmonary infection <4 weeks prior surgery | 40 (4%) | 35 (7%) | 0.010 |
| CRP > 3 mg/dl | 811 (85%) | 427 (91%) | 0.004 |
| Haemoglobin (mg/dl) | 12.7 ± 2.0 | 12.7 ± 2.0 | 0.866 |
| pH | 7.43 ± 0.04 | 7.43 ± 0.04 | 0.272 |
| paO2 (mmHg) | 76 ± 10 | 74 ± 14 | 0.723 |
| paCO2 (mmHg) | 37 ± 5 | 37 ± 6 | 0.240 |
| FEV1 < 60% | 118 (13%) | 100 (22%) | <0.001 |
| No PPC (n = 954) | PPC (n = 472) | p-Value | |
|---|---|---|---|
| Surgery-related characteristics | |||
| Duration of surgery ≥ 195 min | 262 (28%) | 183 (39%) | <0.001 |
| Bronchoplasty | 7 (1%) | 2 (0%) | 0.487 |
| Pneumonectomy | 92 (10%) | 22 (5%) | 0.001 |
| Lobectomy | 569 (60%) | 324 (69%) | 0.001 |
| Bi-lobectomy | 50 (5%) | 49 (10%) | <0.001 |
| Segment resection | 89 (9%) | 44 (9%) | 0.997 |
| Wedge resection | 116 (12%) | 19 (4%) | <0.001 |
| Intraoperative blood loss | 429 ± 508 | 684 ± 643 | 0.001 |
| Partial ligation of pulmonary artery | 119 (13%) | 96 (20%) | <0.001 |
| Anaesthesia-related characteristics | |||
| Total intravenous anaesthesia | 430 (45%) | 238 (50%) | 0.057 |
| Intraoperatively infused crystalloid fluid volume ≥ 6 mL/kg/h | 685 (72%) | 397 (84%) | <0.001 |
| Infused colloids | 87 (9%) | 47 (10%) | 0.610 |
| Intraoperative vasopressors | 742 (78%) | 385 (82%) | 0.098 |
| PRBCs | 46 (5%) | 41 (9%) | 0.004 |
| Fresh frozen plasma | 12 (1%) | 15 (3%) | 0.012 |
| Continuous epidural or paravertebral analgesia | 583 (61%) | 249 (53%) | 0.003 |
| Partial ligation of pulmonary artery | 119 (13%) | 96 (20%) | <0.001 |
| OLV PEEP ≤ 5 cmH2O | 505 (56%) | 286 (64%) | 0.002 |
| OLV PEEP ≤ 7 cmH2O | 799 (88%) | 390 (88%) | 0.851 |
| OLV duration ≥ 175 min | 294 (31%) | 189 (40%) | 0.001 |
| No extubation immediately after surgery | 31 (3%) | 48 (10%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baar, W.; Semmelmann, A.; Knoerlein, J.; Weber, F.; Heinrich, S.; Loop, T., on behalf of Working Group of the German Thorax Registry. Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry. J. Clin. Med. 2022, 11, 5774. https://doi.org/10.3390/jcm11195774
Baar W, Semmelmann A, Knoerlein J, Weber F, Heinrich S, Loop T on behalf of Working Group of the German Thorax Registry. Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry. Journal of Clinical Medicine. 2022; 11(19):5774. https://doi.org/10.3390/jcm11195774
Chicago/Turabian StyleBaar, Wolfgang, Axel Semmelmann, Julian Knoerlein, Frederike Weber, Sebastian Heinrich, and Torsten Loop on behalf of Working Group of the German Thorax Registry. 2022. "Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry" Journal of Clinical Medicine 11, no. 19: 5774. https://doi.org/10.3390/jcm11195774
APA StyleBaar, W., Semmelmann, A., Knoerlein, J., Weber, F., Heinrich, S., & Loop, T., on behalf of Working Group of the German Thorax Registry. (2022). Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry. Journal of Clinical Medicine, 11(19), 5774. https://doi.org/10.3390/jcm11195774





