APOL4, a Novel Immune-Related Prognostic Biomarker for Glioma
Abstract
1. Introduction
2. Methods
2.1. Data Obtain and APOL4 Expression Analysis
2.2. Analyze the Prognostic Value of APOL4 in Glioma
2.3. Evaluation of the Immune Infiltration and the Microenvironment
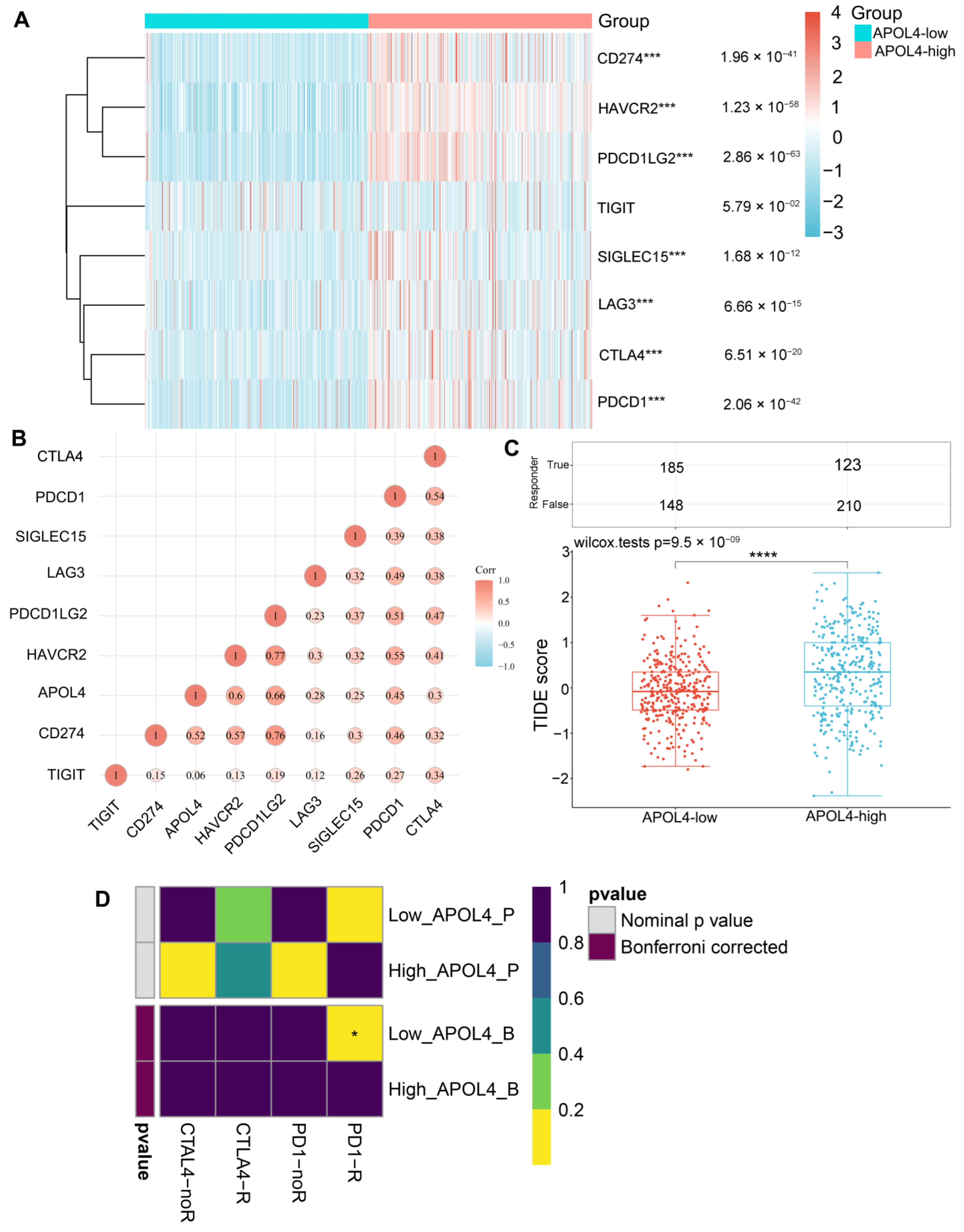
2.4. Assessment of Immune Checkpoints and ICI
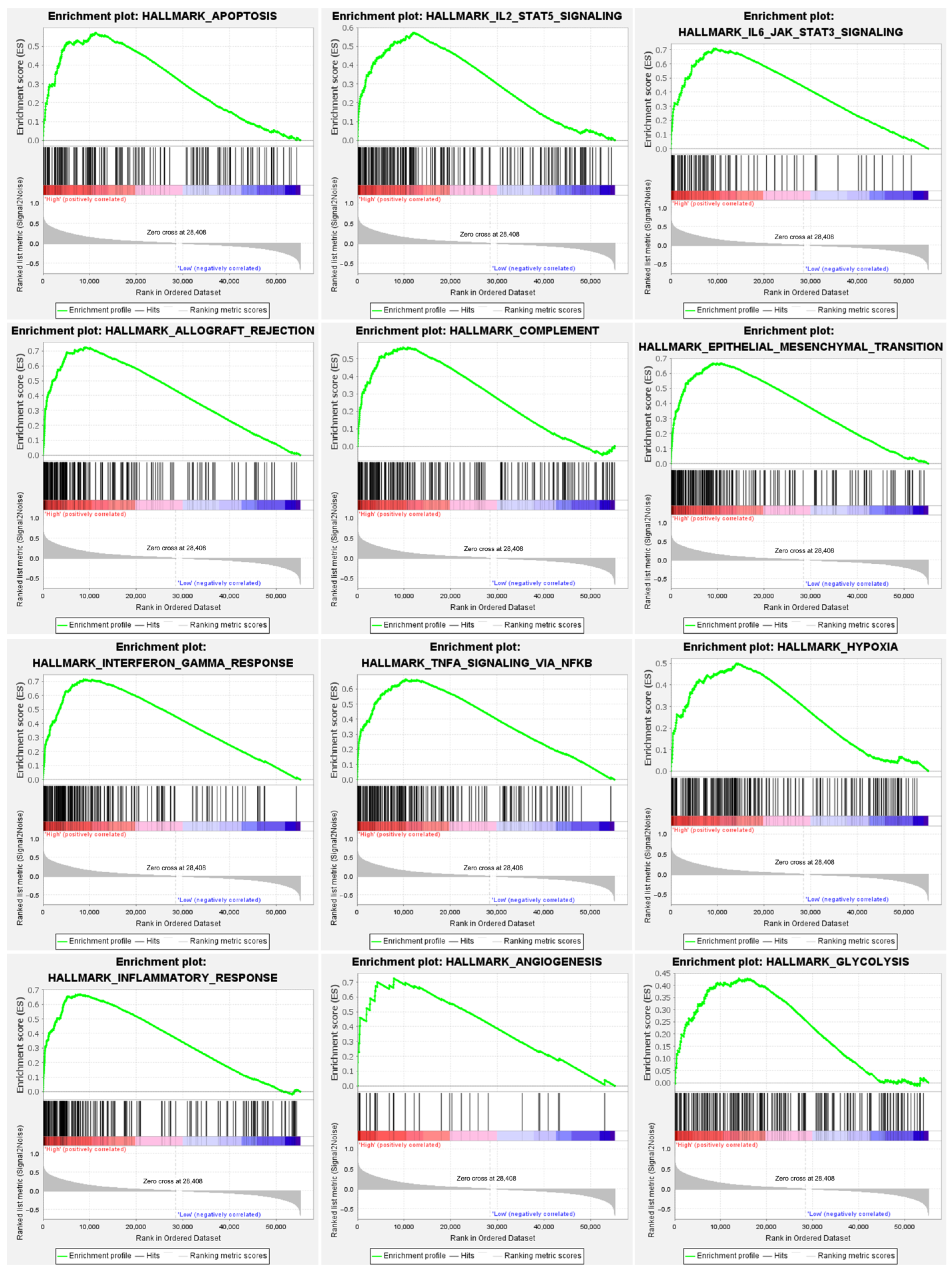
2.5. Potential Function Analysis of APOL4 in Glioma
3. Results
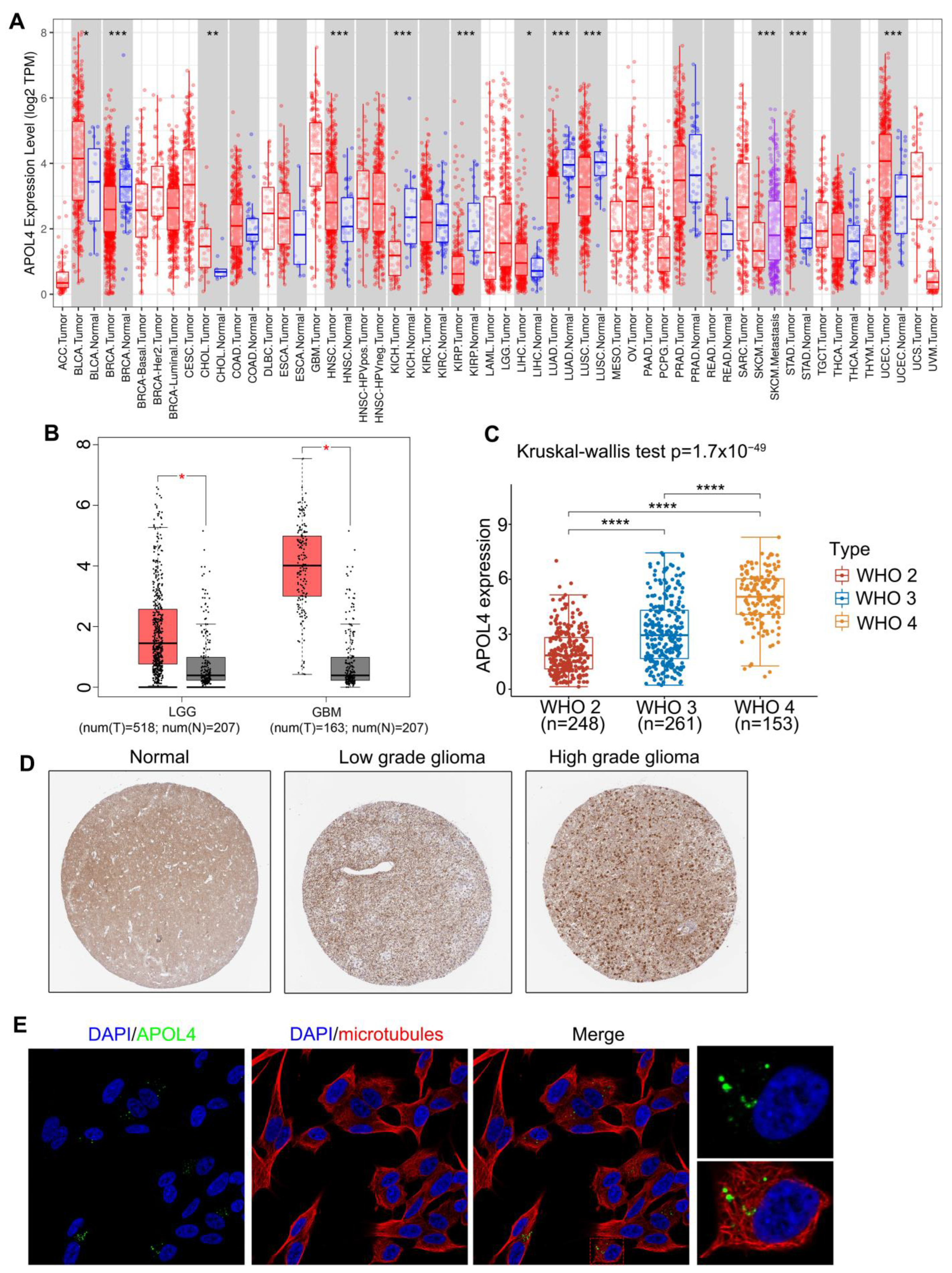
3.1. APOL4 Was Upregulated in Glioma
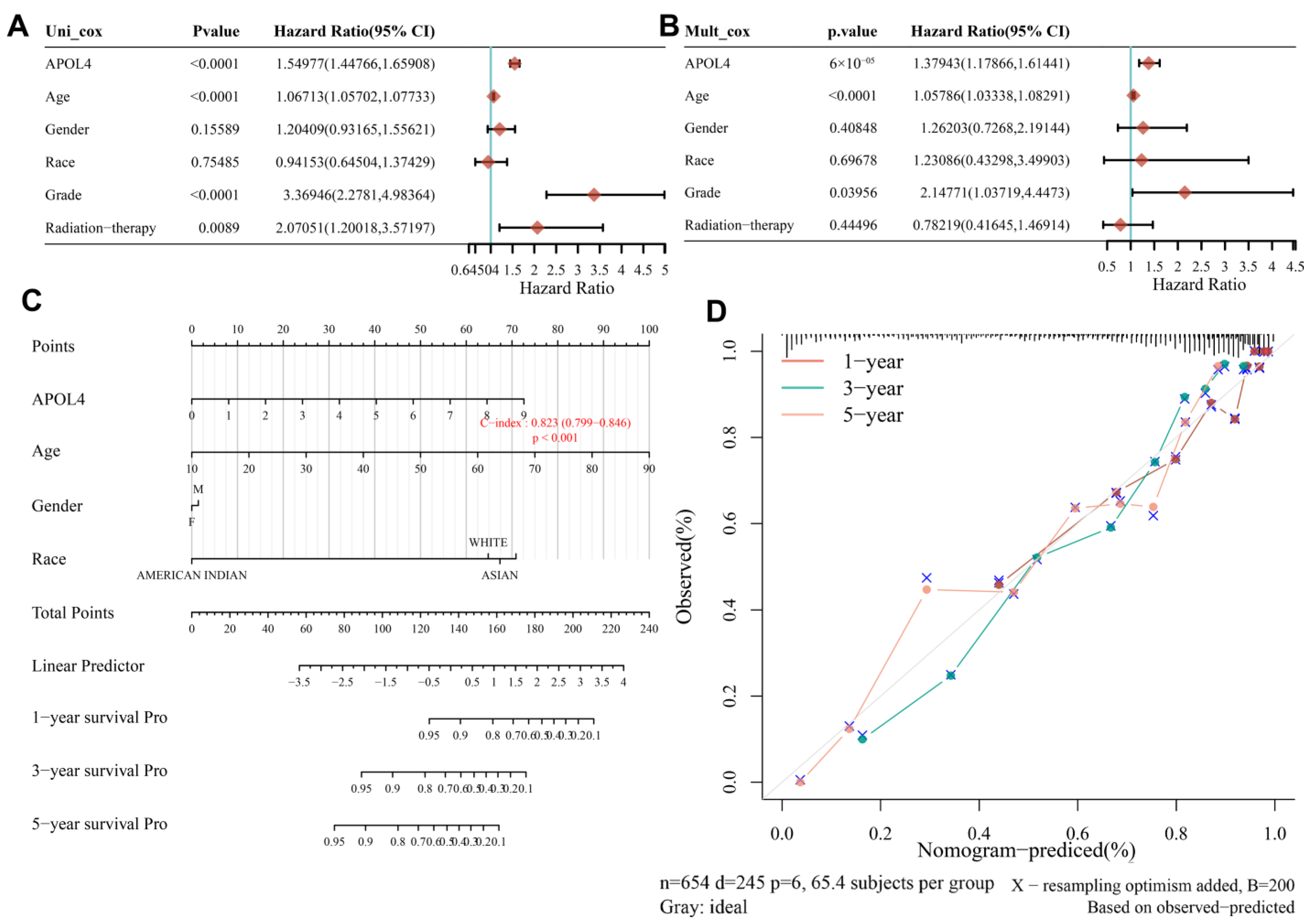
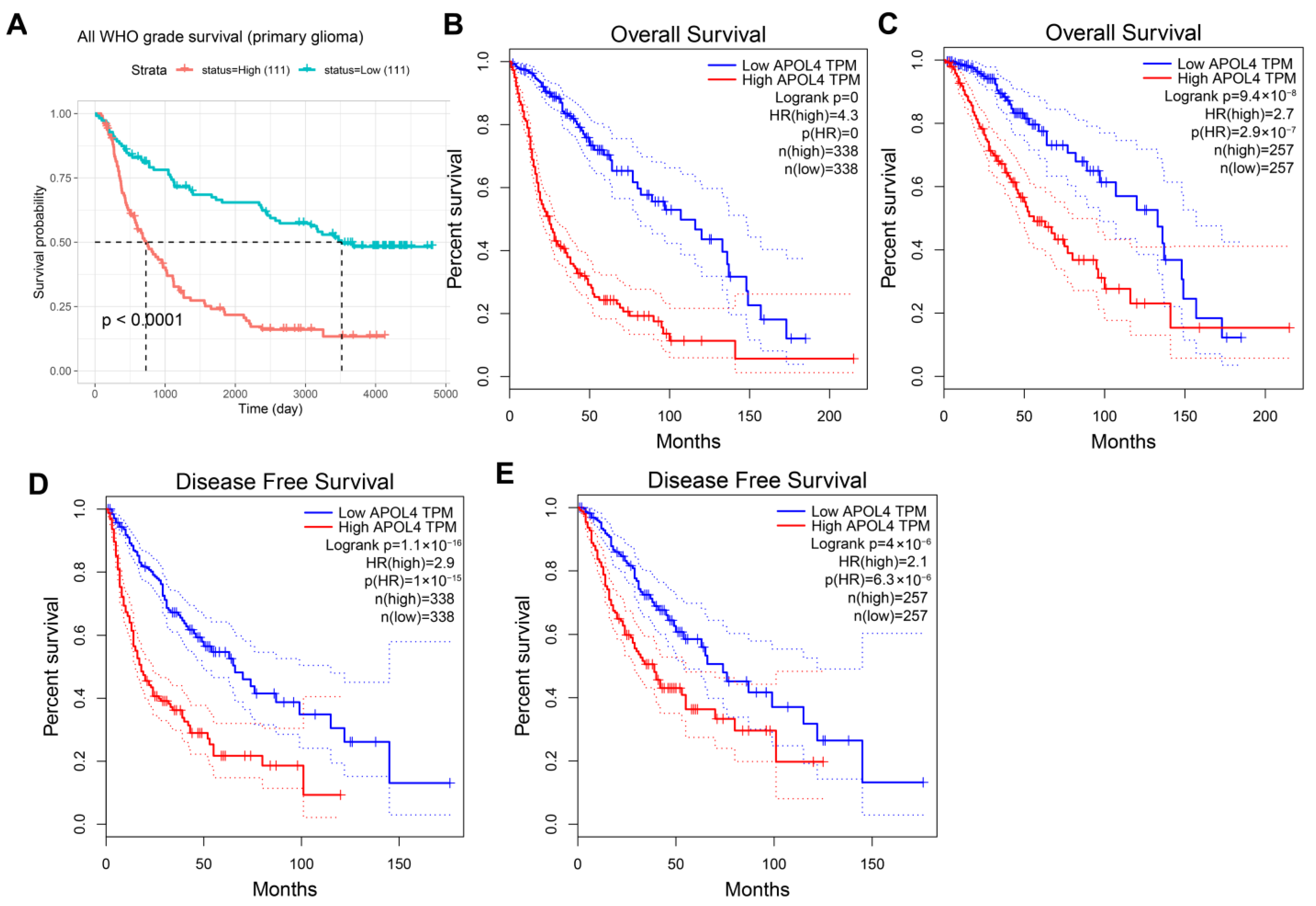
3.2. APOL4 Was an Independent Prognostic Factor
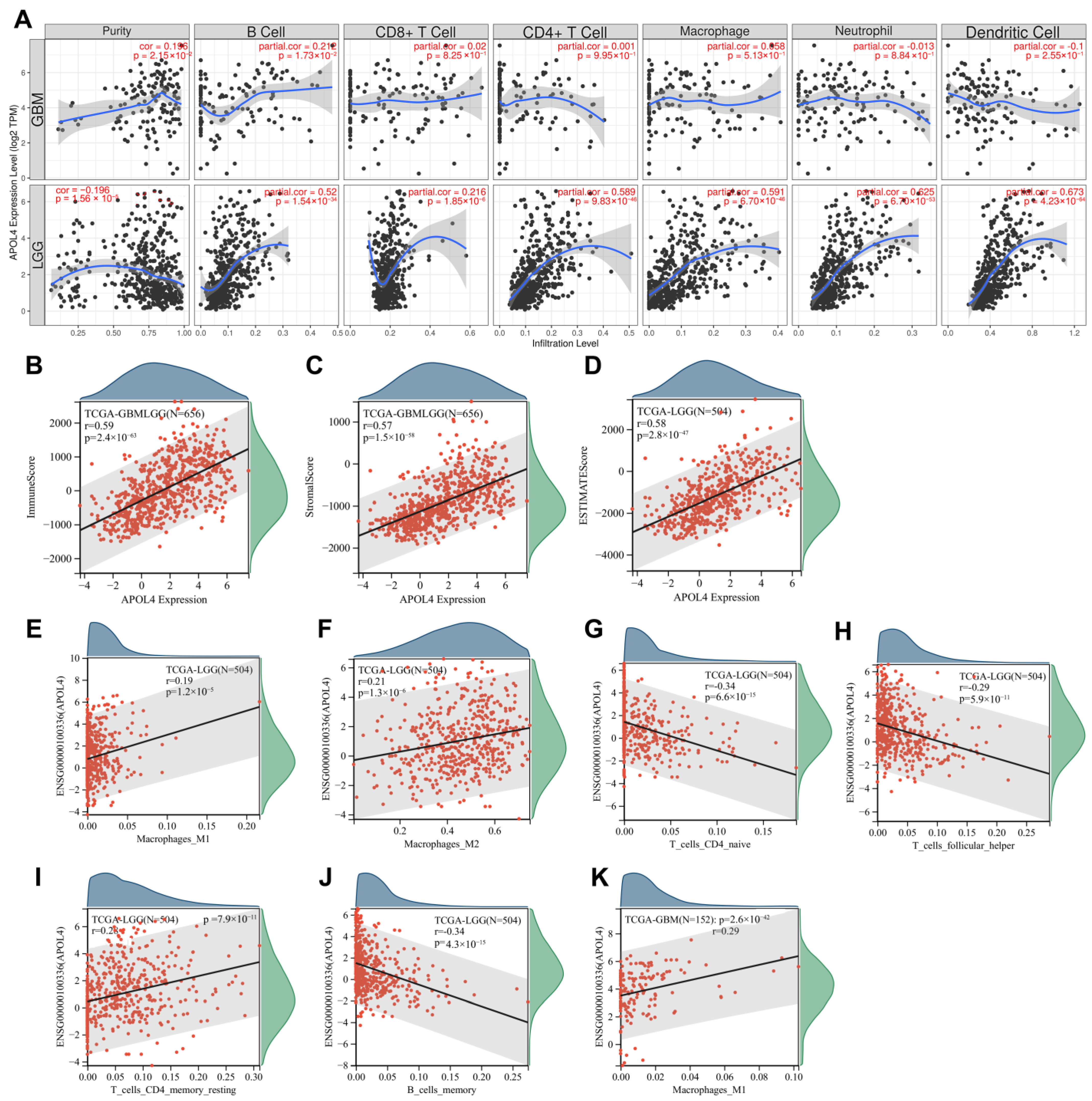
3.3. APOL4 Was Correlated with TIICs Infiltration in Glioma
3.4. APOL4 Was Correlated with the Response to ICI in Glioma Patients
3.5. The Potential Role of APOL4 in Gliomas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusyatiner, O.; Hegi, M.E. Glioma epigenetics: From subclassification to novel treatment options. Semin. Cancer Biol. 2018, 51, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Wu, M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018, 17, 61. [Google Scholar] [CrossRef]
- Sokratous, G.; Polyzoidis, S.; Ashkan, K. Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum. Vaccin. Immunother. 2017, 13, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Pant, J.; Giovinazzo, J.A.; Tuka, L.S.; Peña, D.; Raper, J.; Thomson, R. Apolipoproteins L1-6 share key cation channel-regulating residues but have different membrane insertion and ion conductance properties. J. Biol. Chem. 2021, 297, 100951. [Google Scholar] [CrossRef] [PubMed]
- Ultsch, M.; Holliday, M.J.; Gerhardy, S.; Moran, P.; Scales, S.J.; Gupta, N.; Oltrabella, F.; Chiu, C.; Fairbrother, W.; Eigenbrot, C.; et al. Structures of the ApoL1 and ApoL2 N-terminal domains reveal a non-classical four-helix bundle motif. Commun. Biol. 2021, 4, 916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F. Identification of Ten-Gene Related to Lipid Metabolism for Predicting Overall Survival of Breast Invasive Carcinoma. Contrast Media Mol. Imaging 2022, 2022, 8348780. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, H.; Zhang, X.; He, X.; Xu, X. Comprehensive analysis of pan-cancer reveals potential of ASF1B as a prognostic and immunological biomarker. Cancer Med. 2021, 10, 6897–6916. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, X.; Gu, L.; Jian, Z.; Li, L.; Hu, S.; Qiu, S.; Xiong, X. TUBA1C is a Prognostic Marker in Low-grade Glioma and Correlates with Immune Cell Infiltration in the Tumor Microenvironment. Front. Genet. 2021, 12, 759953. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, X.; Ye, Y.; Jian, Z.; Zhong, Y.; Gu, L.; Xiong, X. Pan-Cancer Analysis of PIMREG as a Biomarker for the Prognostic and Immunological Role. Front. Genet. 2021, 12, 687778. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, X.; Feng, S.; Gu, L.; Jian, Z.; Zou, N.; Xiong, X. Predictive value of PIMREG in the prognosis and response to immune checkpoint blockade of glioma patients. Front. Immunol. 2022, 13, 946692. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, X.; Feng, S.; Jian, Z.; Xu, X.; Gu, L.; Xiong, X. The Hypoxia-Related Gene COL5A1 Is a Prognostic and Immunological Biomarker for Multiple Human Tumors. Oxid Med. Cell. Longev. 2022, 2022, 6419695. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, H.; Chen, B.; He, X.; Shen, Y.; Zhang, X.; Chen, W.; Liu, X.; Xu, Y.; Xu, X. Tubulin Alpha 1b Is Associated with the Immune Cell Infiltration and the Response of HCC Patients to Immunotherapy. Diagnostics 2022, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, T.; Han, J.; Xu, X.; Wu, J.; Song, W.; Liu, G.; Zhu, H.; Zeng, Z. Prognostic Value and Immunological Role of KIFC1 in Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 799651. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, L.; Zhang, L.; Zhu, Y.; Hu, W.; Wang, J.; Ruan, X.; Xu, Z.; Meng, X.; Gao, J.; et al. Immune Signature-Based Subtypes of Cervical Squamous Cell Carcinoma Tightly Associated with Human Papillomavirus Type 16 Expression, Molecular Features, and Clinical Outcome. Neoplasia 2019, 21, 591–601. [Google Scholar] [CrossRef]
- Huai, Q.; Guo, W.; Han, L.; Kong, D.; Zhao, L.; Song, P.; Peng, Y.; Gao, S. Identification of prognostic genes and tumor-infiltrating immune cells in the tumor microenvironment of esophageal squamous cell carcinoma and esophageal adenocarcinoma. Transl. Cancer Res. 2021, 10, 1787–1803. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The Immunology of Hormone Receptor Positive Breast Cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.N.; Chai, R.C.; Wang, K.Y.; Huang, R.Y.; Li, G.Z.; Wang, Y.Z.; Chen, J.; Jiang, T. ADAMTSL4, a Secreted Glycoprotein, Is a Novel Immune-Related Biomarker for Primary Glioblastoma Multiforme. Dis. Markers. 2019, 2019, 1802620. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Hu, X.; Feng, S.; Li, Y.; Zhang, Y.; Qiu, S.; Chen, R.; Ye, Y.; Gu, L.; Jian, Z.; et al. APOL4, a Novel Immune-Related Prognostic Biomarker for Glioma. J. Clin. Med. 2022, 11, 5765. https://doi.org/10.3390/jcm11195765
Zhu H, Hu X, Feng S, Li Y, Zhang Y, Qiu S, Chen R, Ye Y, Gu L, Jian Z, et al. APOL4, a Novel Immune-Related Prognostic Biomarker for Glioma. Journal of Clinical Medicine. 2022; 11(19):5765. https://doi.org/10.3390/jcm11195765
Chicago/Turabian StyleZhu, Hua, Xinyao Hu, Shi Feng, Yuntao Li, Yonggang Zhang, Sheng Qiu, Ran Chen, Yingze Ye, Lijuan Gu, Zhihong Jian, and et al. 2022. "APOL4, a Novel Immune-Related Prognostic Biomarker for Glioma" Journal of Clinical Medicine 11, no. 19: 5765. https://doi.org/10.3390/jcm11195765
APA StyleZhu, H., Hu, X., Feng, S., Li, Y., Zhang, Y., Qiu, S., Chen, R., Ye, Y., Gu, L., Jian, Z., Xu, X., & Xiong, X. (2022). APOL4, a Novel Immune-Related Prognostic Biomarker for Glioma. Journal of Clinical Medicine, 11(19), 5765. https://doi.org/10.3390/jcm11195765






