Left Ventricular Diastolic Indices and Their Impact on Outcomes in Patients with Recently Diagnosed Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Assessment of LVDD
- -
- A score of 1 was assigned if E/e’ ratio ≤8 or DT > 200 ms
- -
- A score of 2 was assigned if E/e’ ratio 9–12 or DT 160–200 ms
- -
- A score of 3 was assigned if E/e’ ratio ≥13 or DT < 160 ms
2.3. Study Outcomes
2.4. Assessment of Patients’ Health Status
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
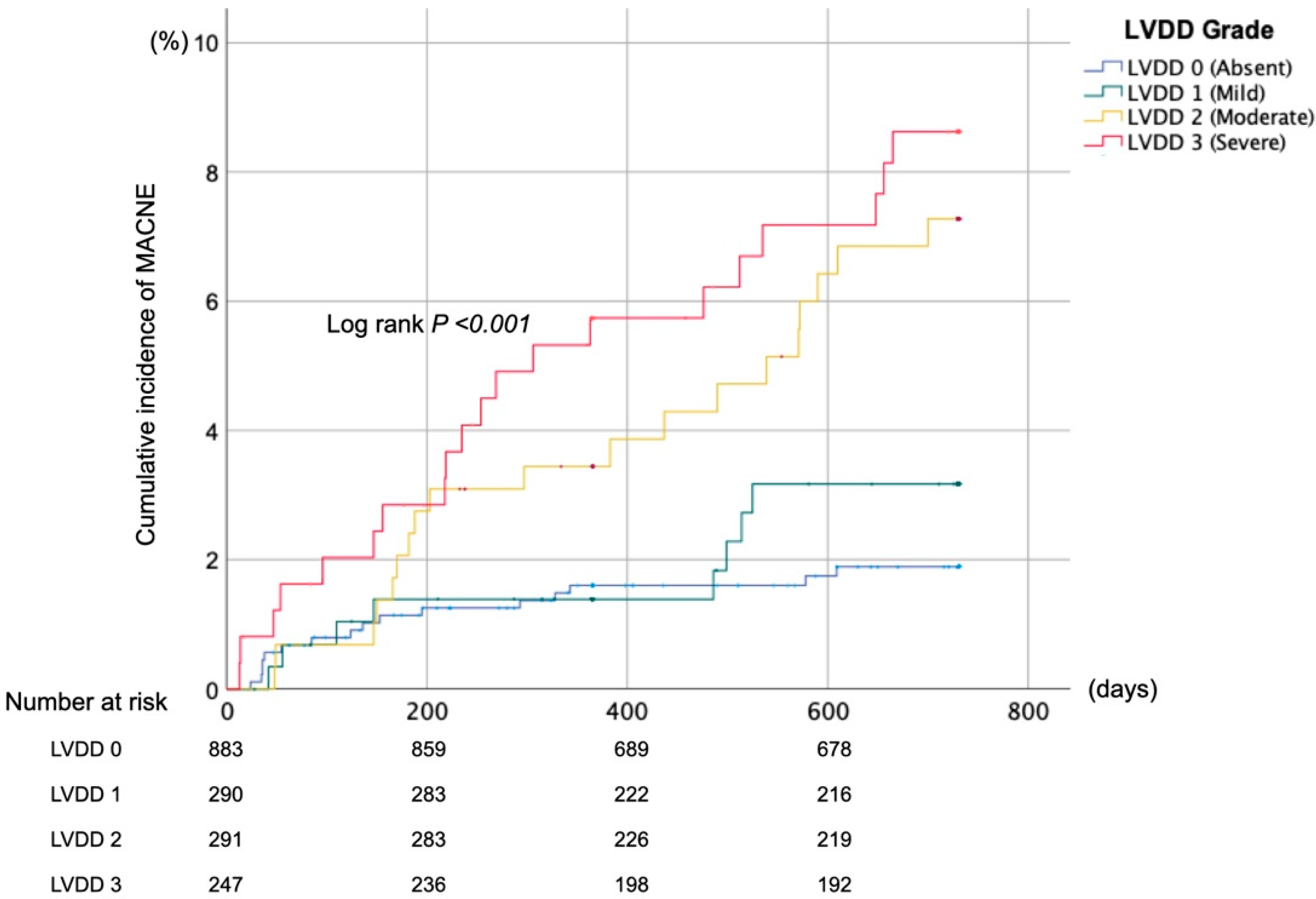
3.2. Composite Clinical Outcomes
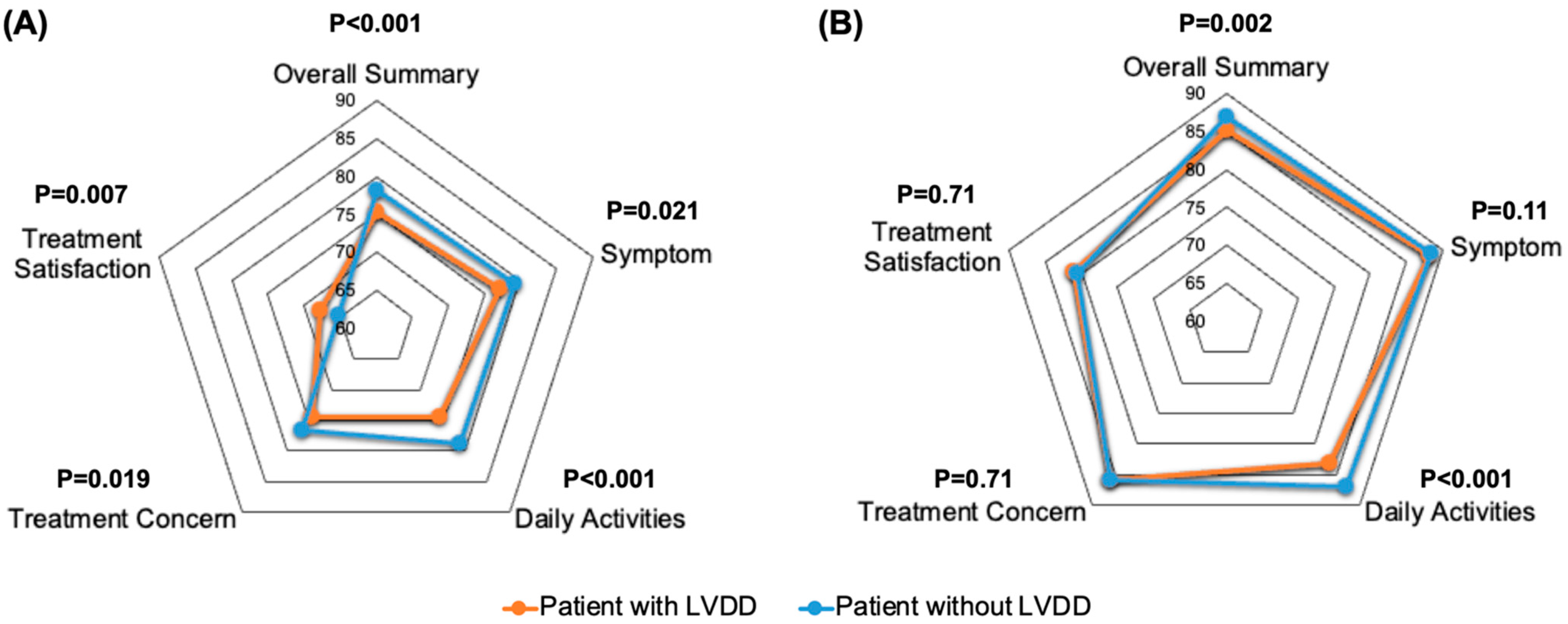
3.3. Health Status Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikemura, N.; Kohsaka, S.; Kimura, T.; Ueda, I.; Katsumata, Y.; Nishiyama, T.; Aizawa, Y.; Tanimoto, K.; Momiyama, Y.; Akaishi, M.; et al. Assessment of Sex Differences in the Initial Symptom Burden, Applied Treatment Strategy, and Quality of Life in Japanese Patients With Atrial Fibrillation. JAMA Netw. Open 2019, 2, e191145. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, N.; Spertus, J.A.; Kimura, T.; Mahaffey, K.; Piccini, J.P.; Inohara, T.; Ueda, I.; Tanimoto, K.; Suzuki, M.; Nakamura, I.; et al. Cohort profile: Patient characteristics and quality-of-life measurements for newly-referred patients with atrial fibrillation-Keio interhospital Cardiovascular Studies-atrial fibrillation (KiCS-AF). BMJ Open 2019, 9, e032746. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Zareian, M.; Ambale Venkatesh, B.; Samiei, S.; Imai, M.; Wu, C.; Launer, L.J.; Shea, S.; Gottesman, R.F.; Heckbert, S.R.; et al. Left Atrial Mechanical Function and Incident Ischemic Cerebrovascular Events Independent of AF: Insights From the MESA Study. JACC Cardiovasc. Imaging 2019, 12, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.; Dorian, P.; Bubien, R.; Lewis, S.; Godejohn, D.; Reynolds, M.R.; Lakkireddy, D.R.; Wimmer, A.P.; Bhandari, A.; Burk, C. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wynn, G.J.; Todd, D.M.; Webber, M.; Bonnett, L.; McShane, J.; Kirchhof, P.; Gupta, D. The European Heart Rhythm Association symptom classification for atrial fibrillation: Validation and improvement through a simple modification. Europace 2014, 16, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, N.; Kimura, T.; Sawano, M.; Fukuda, K.; Takatsuki, S.; Spertus, J.A.; Kohsaka, S. Novel Approach for Visualizing Multiple Domains of Quality of Life Scales. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005573. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, N.; Spertus, J.A.; Kimura, T.; Katsumata, Y.; Fujisawa, T.; Ueda, I.; Fukuda, K.; Takatsuki, S.; Kohsaka, S. Baseline and Postprocedural Health Status Outcomes in Contemporary Patients With Atrial Fibrillation Who Underwent Catheter Ablation: A Report from the Japanese Outpatient Registry. J. Am. Heart Assoc. 2021, 10, e019983. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Lam, C.S.P.; Lund, L.H.; Redfield, M.M. Interdependence of Atrial Fibrillation and Heart Failure With a Preserved Ejection Fraction Reflects a Common Underlying Atrial and Ventricular Myopathy. Circulation 2020, 141, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Benjamin, E.J.; Albert, C.M.; Alonso, A.; Chauhan, C.; Chen, P.S.; Curtis, A.B.; Desvigne-Nickens, P.; Ho, J.E.; Lam, C.S.P.; et al. Advancing Research on the Complex Interrelations Between Atrial Fibrillation and Heart Failure: A Report From a US National Heart, Lung, and Blood Institute Virtual Workshop. Circulation 2020, 141, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kim, S.; Moore, C.; Thomas, L.; Gersh, B.; Allen, L.A.; Kowey, P.R.; Mahaffey, K.W.; Hylek, E.; Peterson, E.D.; et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients With Prevalent Atrial Fibrillation. JACC Heart Fail. 2017, 5, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Barmeyer, A.; Mullerleile, K.; Mortensen, K.; Meinertz, T. Diastolic dysfunction in exercise and its role for exercise capacity. Heart Fail. Rev. 2009, 14, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.G.; Fontes-Carvalho, R.; Sampaio, F.; Ribeiro, J.; Bettencourt, P.; Flachskampf, F.A.; Leite-Moreira, A.; Azevedo, A. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J. Cardiovasc. Imaging 2018, 19, 380–386. [Google Scholar] [CrossRef] [PubMed]
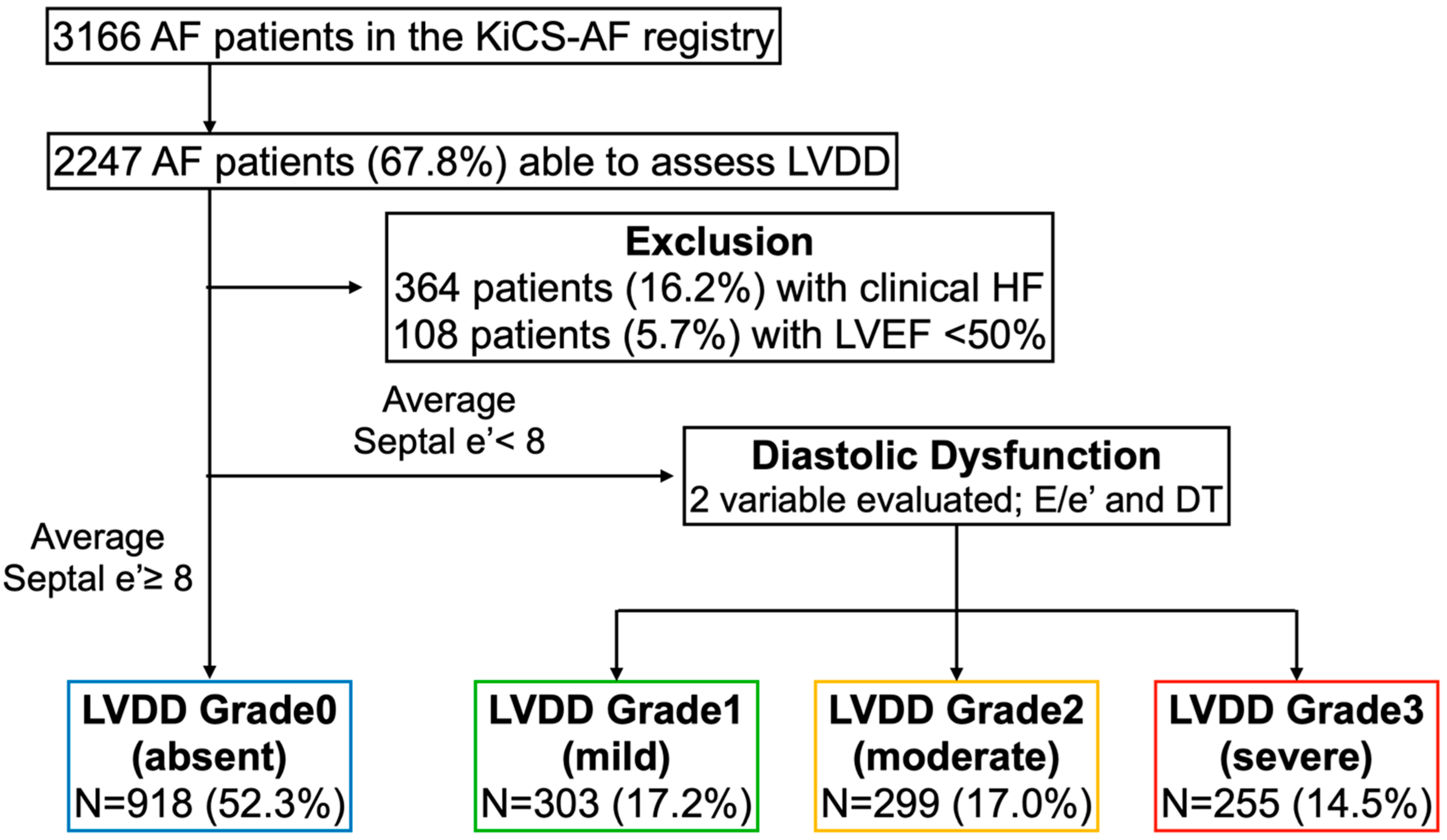
| Characteristics, n (%) | Patients with Normal Diastolic Function (Grade 0) n = 918 | Patients with Mild Diastolic Dysfunction (Grade 1) n = 303 | Patients with Moderate Diastolic Dysfunction (Grade 2) n = 299 | Patient with Severe Diastolic Dysfunction (Grade 3) n = 255 | p Value |
|---|---|---|---|---|---|
| Age, mean, years (SD) | 62.8 (11.5) | 68.8 (9.2) | 70.3 (9.8) | 71.6 (9.7) | <0.001 |
| Men | 683 (74.4) | 207 (68.3) | 188 (62.9) | 155 (60.8) | <0.001 |
| BMI, median, kg/m2 (SD) | 23.8 (3.5) | 23.5 (3.4) | 23.5 (3.6) | 23.5 (3.3) | 0.159 |
| Heart rate, mean, bpm (SD) | 79.4 (17.6) | 73.7 (15.0) | 76.9 (16.0) | 79.8 (18.9) | 0.052 |
| Blood pressure, mean, mmHg (SD) | |||||
| Systolic | 130.2 (18.8) | 134.2 (19.8) | 133.4 (18.4) | 133.3 (18.5) | 0.002 |
| Diastolic | 79 (13.2) | 76.8 (13.5) | 76.8 (13.5) | 77.4 (12.5) | 0.039 |
| Medical history | |||||
| Smoking | 155 (16.9) | 46 (15.2) | 44 (14.7) | 28 (11.0) | 0.13 |
| Hypertension | 419 (45.6) | 189 (62.4) | 196 (65.6) | 168 (65.9) | <0.001 |
| Diabetes mellitus | 103 (11.2) | 42 (13.9) | 50 (16.7) | 52 (20.4) | <0.001 |
| Dyslipidemia | 257 (28.0) | 122 (40.3) | 124 (41.5) | 97 (38.0) | <0.001 |
| Stroke or TIA | 56 (6.1) | 28 (9.2) | 29 (9.7) | 23 (9.0) | 0.084 |
| CKD (eGFR < 60 mL/min) | 297 (32.4) | 121 (39.9) | 121 (40.5) | 123 (48.2) | <0.001 |
| Peripheral artery disease | 18 (2.0) | 12 (4.0) | 12 (4.0) | 10 (3.9) | 0.099 |
| Coronary artery disease | 27 (2.9) | 15 (5.0) | 21 (7.0) | 24 (9.4) | <0.001 |
| Pacemaker implantation | 5 (0.5) | 4 (1.3) | 8 (2.7) | 5 (2.0) | 0.021 |
| Echocardiographic Parameters | |||||
| Ejection Fraction, % (SD) | 60.2 (3.7) | 60.1 (3.5) | 60.3 (3.8) | 60.2 (3.8) | 0.28 |
| LV Hypertrophy | 33 (3.6) | 11 (3.6) | 27 (9.0) | 19 (7.5) | <0.001 |
| LA diameter, mm (SD) | 40 (7) | 37 (6) | 40 (7) | 41 (7) | 0.20 |
| Average e’, cm/s (SD) | 10.3 (2.0) | 6.4 (1.1) | 5.9 (1.3) | 6.3 (1.2) | <0.001 |
| E/e’ ratio (SD) | 8 (2.4) | 9.2 (2.0) | 13.5 (5.1) | 15 (5.4) | <0.001 |
| Deceleration time, msec (SD) | 181.3 (48.5) | 243.6 (48.3) | 210.6 (49.7) | 151.7 (26.1) | 0.61 |
| Moderate or severe MS | 0 | 0 | 1(0.1) | 0 | 0.34 |
| Moderate or severe MR | 37 (4.0) | 6 (2.0) | 21 (7.0) | 28 (11.0) | <0.001 |
| Type of visit | |||||
| Referral from emergency department | 46 (5.0) | 33 (10.9) | 29 (9.7) | 26 (10.2) | <0.001 |
| Diagnosed at health screening | 336 (36.6) | 53 (17.5) | 63 (21.1) | 61 (23.9) | <0.001 |
| Type of AF at registration | |||||
| First detected | 39 (4.2) | 20 (6.6) | 21 (7.0) | 11 (4.3) | <0.001 |
| Paroxysmal | 434 (47.3) | 249 (82.5) | 194 (64.9) | 109 (42.9) | |
| Persistent | 291 (31.7) | 21 (7.0) | 58 (19.4) | 87 (34.3) | |
| Permanent | 136 (14.8) | 10 (3.3) | 25 (8.4) | 45 (17.7) | |
| Current drug therapy | |||||
| β-blockers | 430 (46.8) | 131 (43.2) | 161 (53.8) | 142 (55.7) | 0.005 |
| ACE inhibitors/ARBs | 239 (26.0) | 121 (39.9) | 124 (41.5) | 97 (38.0) | <0.001 |
| Calcium-channel blockers | 330 (35.9) | 127 (41.9) | 137 (45.8) | 134 (52.5) | <0.001 |
| Digoxin | 27 (2.9) | 5 (1.7) | 10 (3.3) | 10 (3.9) | 0.42 |
| Diuretics | 60 (6.5) | 18 (5.9) | 31 (10.4) | 37 (14.5) | <0.001 |
| Currently using antiarrhythmic drugs | 216 (23.5) | 101 (33.3) | 80 (26.8) | 41 (16.1) | <0.001 |
| Oral anticoagulants | |||||
| None | 190 (20.7) | 56 (18.5) | 47 (15.7) | 16 (6.3) | <0.001 |
| Warfarin | 86 (9.4) | 28 (9.2) | 29 (9.7) | 37 (14.5) | 0.099 |
| Direct oral anticoagulants | 643 (70.0) | 219 (72.3) | 223 (74.6) | 202 (79.2) | 0.027 |
| Prior interventional therapy for AF | |||||
| Catheter ablation of AF | 50 (5.4) | 37 (12.2) | 31 (10.4) | 25 (9.8) | <0.001 |
| Surgical maze | 1 (0.1) | 0 (0.0) | 2 (0.7) | 1 (0.4) | 0.24 |
| BNP, mean, pg/mL, (SD) | 105.3 (107.5) | 77 (123.9) | 139.7 (162.2) | 171.9 (137.9) | <0.001 |
| CHA2DS2-VASc score, (SD) | 1.6 (1.4) | 2.3 (1.4) | 2.6 (1.5) | 2.7 (1.3) | <0.001 |
| Outcomes | Patients with Normal Diastolic Function (Grade 0) n = 918 | Patients with Mild Diastolic Dysfunction (Grade 1) n = 303 | Patients with Moderate Diastolic Dysfunction (Grade 2) n = 299 | Patient with Severe Diastolic Dysfunction (Grade 3) n = 255 | p Value | |
|---|---|---|---|---|---|---|
| MACNE | Incidence, n (%) | 16 (1.8) | 8 (2.8) | 19 (6.5) | 20 (8.1) | <0.001 |
| Adjusted HRs (95%CI) | Reference | 1.10 (0.44–2.76) | 1.82 (0.89–3.71) | 2.28 (1.13–4.60) | - | |
| All-cause death | Incidence, n (%) | 3 (0.3) | 3 (1.0) | 10 (3.4) | 5 (2.0) | <0.001 |
| Adjusted HRs (95%CI) | Reference | 2.46 (0.44–13.5) | 4.66 (1.21–17.9) | 2.27 (0.50–10.1) | - | |
| Heart failure hospitalization | Incidence, n (%) | 9 (1.0) | 2 (0.7) | 5 (1.7) | 11 (4.5) | 0.001 |
| Adjusted HRs (95%CI) | Reference | 0.39 (0.07–2.03) | 0.71 (0.21–2.34) | 1.78 (0.68–4.69) | - | |
| Stroke | Incidence, n (%) | 5 (0.6) | 3 (1.0) | 4 (1.4) | 1 (0.4) | 0.66 |
| Adjusted HRs (95%CI) | Reference | 1.35 (0.27–6.59) | 1.32 (0.32–5.47) | 0.48 (0.05–4.42) | - | |
| Patients with Normal Diastolic Function (Grade 0) n = 918 (51.7%) | Patients with Diastolic Dysfunction (Any Grade) n = 857 (48.3%) | p Value | |
|---|---|---|---|
| Baseline, mean (SD) | |||
| Overall summary | 78.2 (16.7) | 75.2 (18.3) | <0.001 |
| Symptom | 79.2 (18.8) | 77.1 (19.9) | 0.021 |
| Daily activities | 78.8 (20.7) | 74.5 (22.8) | <0.001 |
| Treatment concerns | 76.6 (17.5) | 74.5 (19.2) | 0.019 |
| Treatment satisfaction | 65.1 (20.8) | 67.7 (20.1) | 0.007 |
| 1-year after registration, mean (SD) | |||
| Overall summary | 87.0 (13.3) | 85.2 (14.4) | 0.002 |
| Symptom | 88.6 (14.8) | 88.3 (15.5) | 0.11 |
| Daily activities | 87.0 (16.8) | 83.2 (18.4) | <0.001 |
| Treatment concerns | 85.8 (13.4) | 85.9 (14.7) | 0.71 |
| Treatment satisfaction | 80.7 (19.2) | 81.2 (18.0) | 0.71 |
| Change within 1-year, mean (95% confidence interval) * | |||
| Overall summary | 9.6 (8.7–10.4) | 8.5 (7.7–9.4) | 0.10 |
| Symptom | 10.2 (9.5–11.2) | 9.6 (8.6–10.6) | 0.38 |
| Daily activities | 9.0 (7.9–10.1) | 6.8 (5.7–7.9) | 0.006 |
| Treatment concerns | 10.1 (9.2–11.0) | 10.6 (9.6–11.5) | 0.50 |
| Treatment satisfaction | 14.5 (13.0– 15.9) | 14.6 (13.1–16.1) | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemura, N.; Nakanishi, K.; Spertus, J.A.; Lam, C.S.P.; Kimura, T.; Katsumata, Y.; Fujisawa, T.; Ueda, I.; Ohki, T.; Fukuda, K.; et al. Left Ventricular Diastolic Indices and Their Impact on Outcomes in Patients with Recently Diagnosed Atrial Fibrillation. J. Clin. Med. 2022, 11, 5732. https://doi.org/10.3390/jcm11195732
Ikemura N, Nakanishi K, Spertus JA, Lam CSP, Kimura T, Katsumata Y, Fujisawa T, Ueda I, Ohki T, Fukuda K, et al. Left Ventricular Diastolic Indices and Their Impact on Outcomes in Patients with Recently Diagnosed Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(19):5732. https://doi.org/10.3390/jcm11195732
Chicago/Turabian StyleIkemura, Nobuhiro, Koki Nakanishi, John A. Spertus, Carolyn S. P. Lam, Takehiro Kimura, Yoshinori Katsumata, Taishi Fujisawa, Ikuko Ueda, Takahiro Ohki, Keiichi Fukuda, and et al. 2022. "Left Ventricular Diastolic Indices and Their Impact on Outcomes in Patients with Recently Diagnosed Atrial Fibrillation" Journal of Clinical Medicine 11, no. 19: 5732. https://doi.org/10.3390/jcm11195732
APA StyleIkemura, N., Nakanishi, K., Spertus, J. A., Lam, C. S. P., Kimura, T., Katsumata, Y., Fujisawa, T., Ueda, I., Ohki, T., Fukuda, K., Takatsuki, S., & Kohsaka, S. (2022). Left Ventricular Diastolic Indices and Their Impact on Outcomes in Patients with Recently Diagnosed Atrial Fibrillation. Journal of Clinical Medicine, 11(19), 5732. https://doi.org/10.3390/jcm11195732






