Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review
Abstract
1. Introduction
2. Case Series
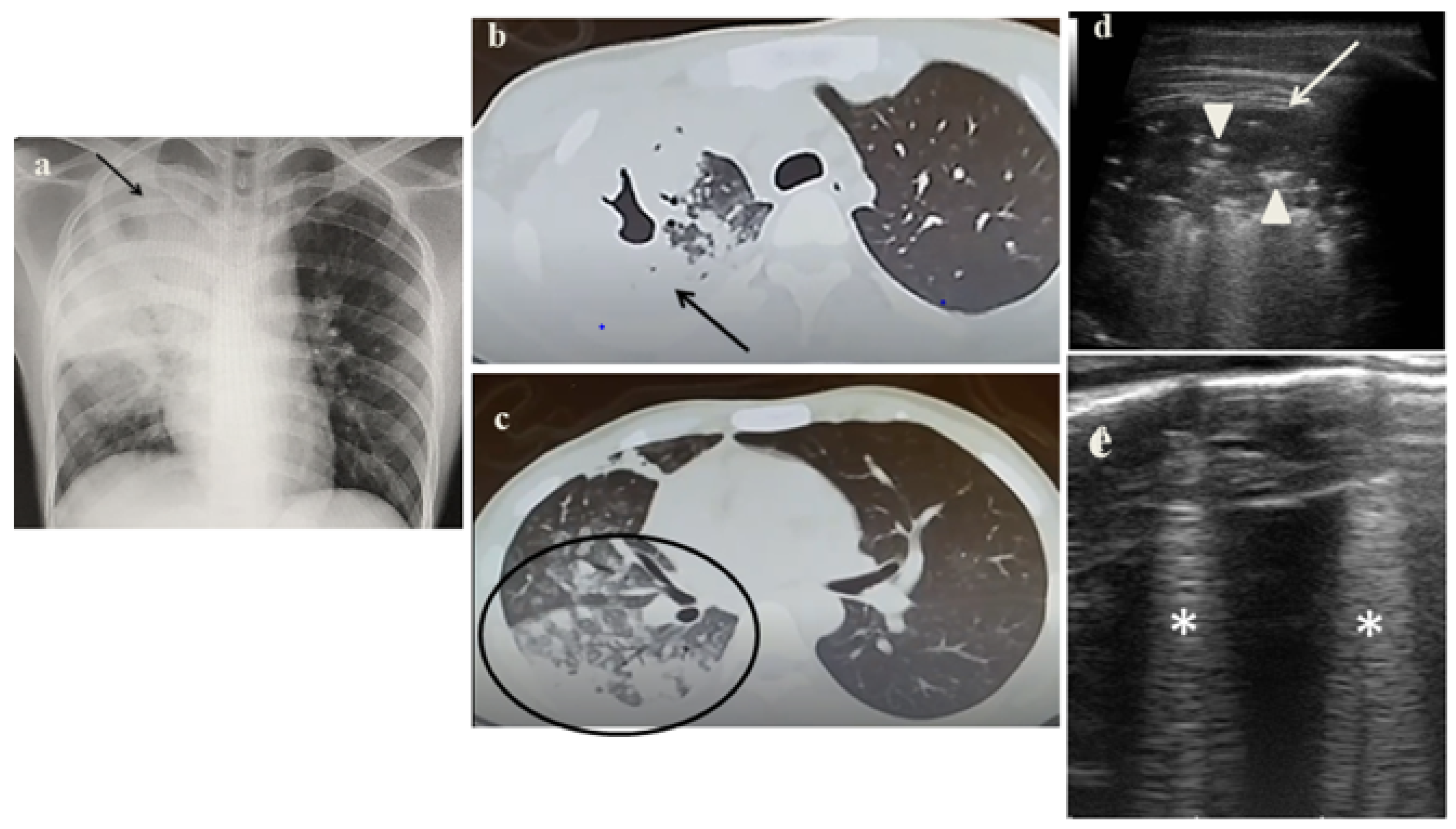
2.1. Case 1
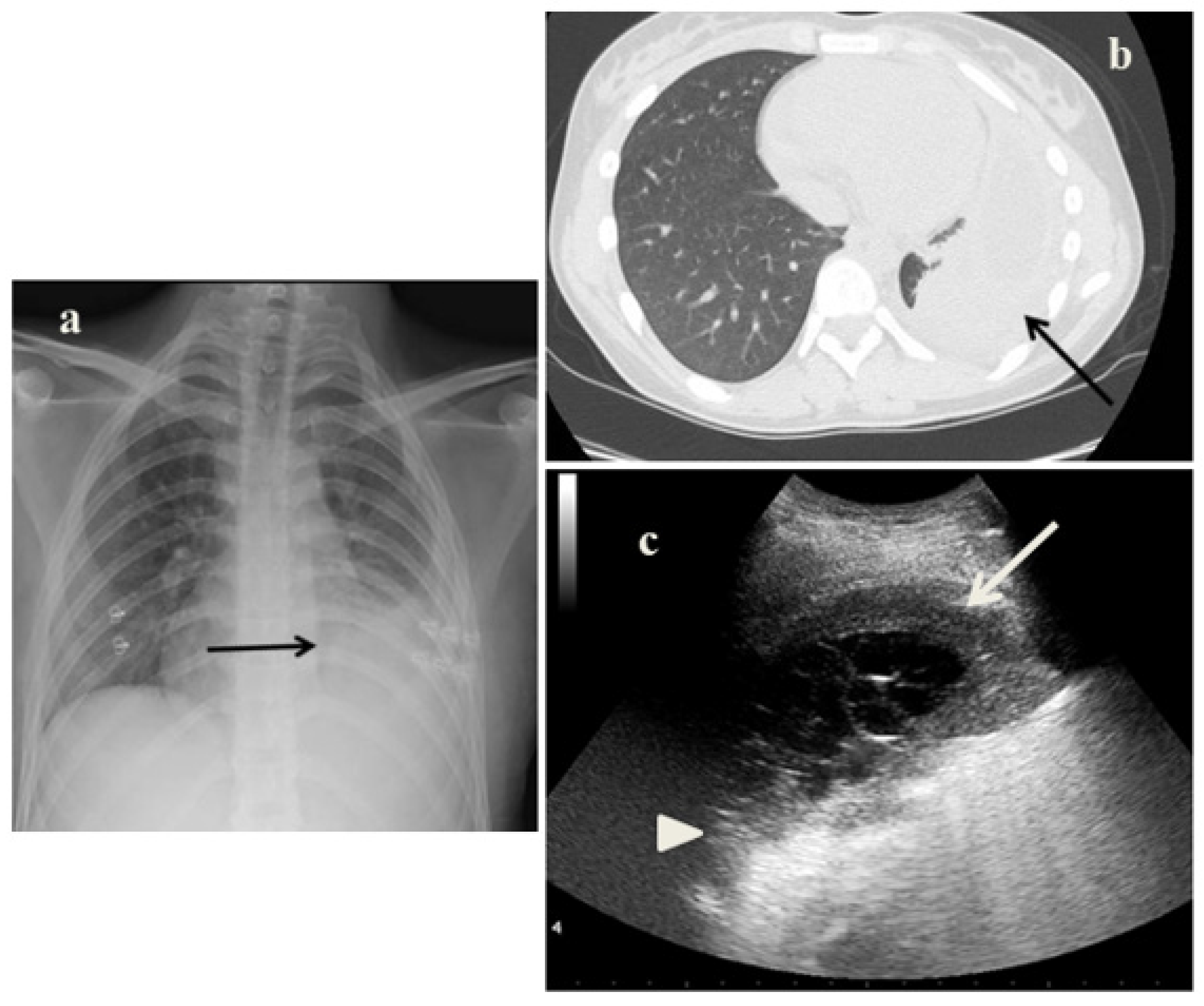
2.2. Case 2
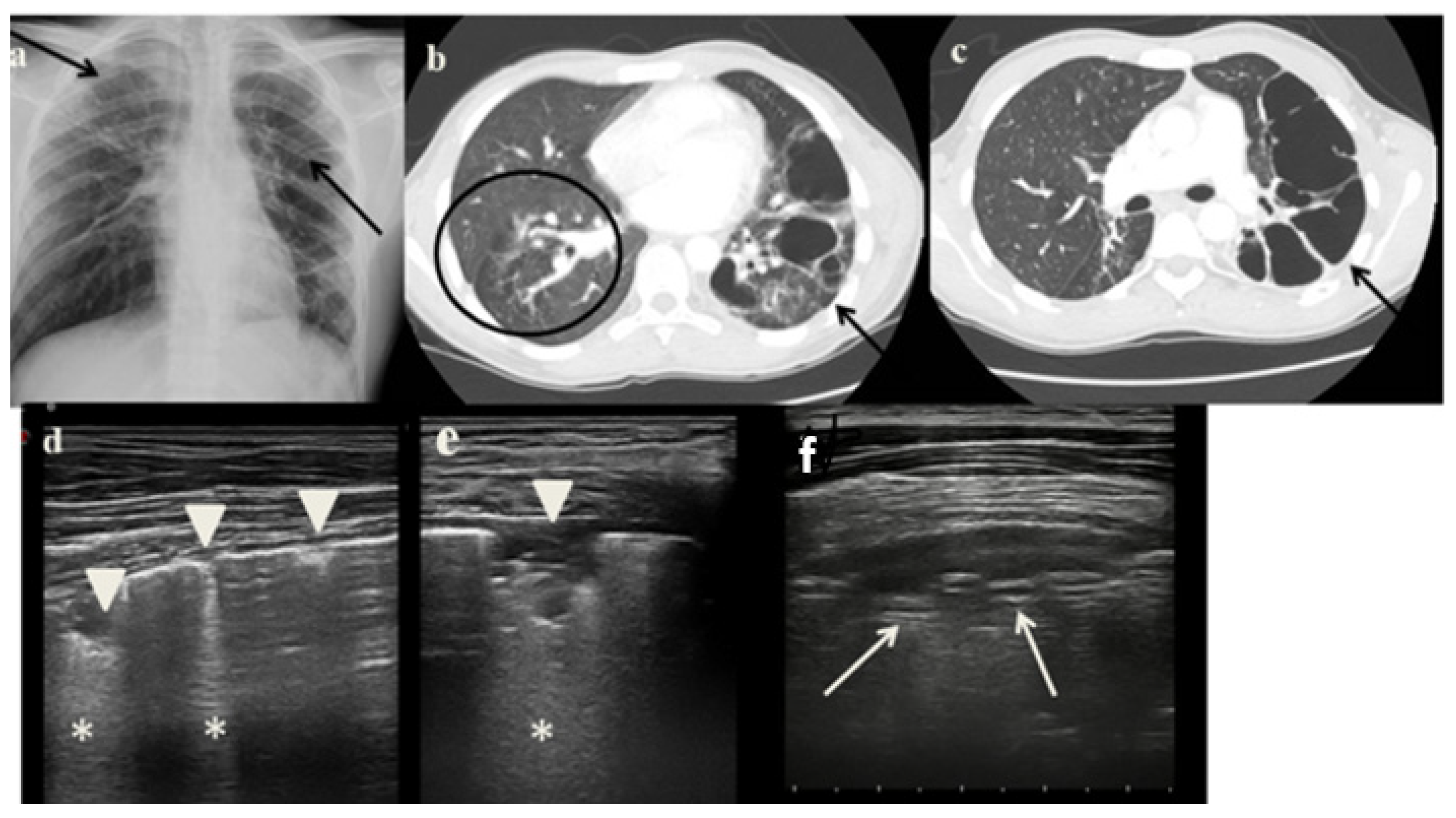
2.3. Case 3
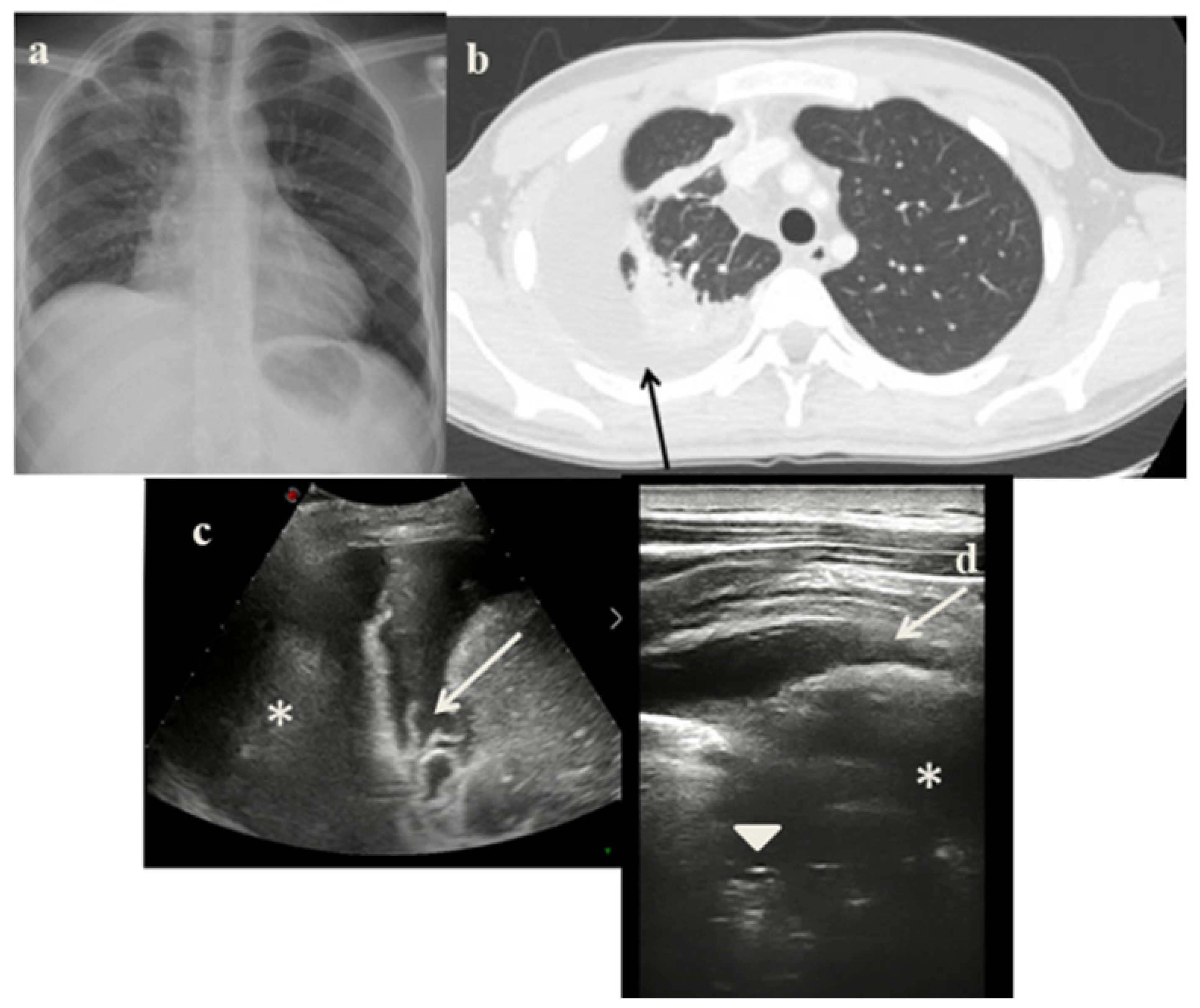
2.4. Case 4
3. Discussion
- It identified in cases 1 and 3 large consolidations (Figure 1c and Figure 3c) with significant air content documented by thickened aerial broncograms. We have noticed that the latter have different characteristics compared to the air bronchograms present in bacterial or viral pneumonia [9]. These large consolidations could represent the tubercular cavitation seen at chest CT, but all these findings still need to be studied to better clarify their meaning;
- In the cases 2 and 4, it demonstrated its superiority compared to CXR in characterizing the pleural effusion and consolidation of an atelectatic nature (Figure 2c and Figure 4c); LUS is therefore useful as a non-invasive diagnostic instrumental support to identify and characterize a picture of tuberculous pleuritis.
- It was not possible to evaluate the hilar lymph nodal disease (Figure 1b), the hallmark of pediatric PTB, because of the interposition of air between the ultrasound and hilar lymph nodes; and
- It was not possible to detect lesions to far from the pleura; in adults, parenchymal lesions are wider and easier to be assessed by ultrasounds [8].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bélard, S.; Heuvelings, C.C.; Banderker, E.; Bateman, L.; Heller, T.; Andronikou, S.; Workman, L.; Grobusch, M.P.; Zar, H.J. Utility of Point-of-care Ultrasound in Children with Pulmonary Tuberculosis. Pediatr. Infect. Dis. J. 2018, 37, 637–642. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Seddon, J.A.; Shingadia, D. Epidemiology and disease burden of tuberculosis in children: A global perspective. Infect. Drug Resist. 2014, 7, 153–165. [Google Scholar] [CrossRef]
- Newton, S.M.; Brent, A.J.; Anderson, S. Paediatric tuberculosis. Lancet Infect. Dis. 2008, 8, 498–510. [Google Scholar] [CrossRef]
- Bates, M.; Shibemba, A.; Mudenda, V.; Chimoga, C.; Tembo, J.; Kabwe, M.; Chilufya, M.; Hoelscher, M.; Maeurer, M.; Sinyangwe, S.; et al. Burden of respiratory tract infections at post mortem in Zambian children. BMC Med. 2016, 14, 99. [Google Scholar] [CrossRef]
- Graham, S.M.; Ahmed, T.; Amanullah, F.; Browning, R.; Cardenas, V.; Casenghi, M.; Cuevas, L.E.; Gale, M.; Gie, R.P.; Grzemska, M.; et al. Evaluation of Tuberculosis Diagnostics in Children: 1. Proposed Clinical Case Definitions for Classification of Intrathoracic Tuberculosis Disease. Consensus from an Expert Panel. J. Infect. Dis. 2012, 205 (Suppl. 2), S199–S208. [Google Scholar] [CrossRef]
- Tomà, P.; Lancella, L.; Menchini, L.; Lombardi, R.; Secinaro, A.; Villani, A. Radiological patterns of childhood thoracic tuberculosis in a developed country: A single institution’s experience on 217/255 cases. Radiol. Med. 2017, 122, 22–34. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pezza, L.; Valentini, P. Utility of Point-of-care Ultrasound in Children with Pulmonary Tuberculosis. Pediatr. Infect. Dis. J. 2018, 37, e280–e281. [Google Scholar] [CrossRef]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: A prospective study. J. Ultrasound 2022, 25, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Pomykala, K.; Desai, I.; Jardon, M.; Naik, P.; Pool, K.-L. Imaging of Tuberculosis in Resource-Limited Settings. Curr. Radiol. Rep. 2019, 7, 23. [Google Scholar] [CrossRef]
- Cattarossi, L.; Copetti, R.; Poskurica, B. Radiation Exposure Early in Life Can Be Reduced by Lung Ultrasound. Chest 2011, 139, 730–731. [Google Scholar] [CrossRef][Green Version]
- Buonsenso, D.; De Rose, C. Implementation of lung ultrasound in low- to middle-income countries: A new challenge global health? Eur. J. Pediatr. 2022, 181, 1–8. [Google Scholar] [CrossRef]
- Volpicelli, G. Lung sonography. J. Ultrasound Med. 2013, 32, 165–171. [Google Scholar] [CrossRef]
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung ultrasound features of children with complicated and non-complicated community acquired pneumonia: A prospective study. Pediatr. Pulmonol. 2019, 54, 1479–1486. [Google Scholar] [CrossRef]
- Tomà, P. Lung ultrasound in pediatric radiology—Cons. Pediatr. Radiol. 2020, 50, 314–320. [Google Scholar] [CrossRef]
- Musolino, A.M.; Supino, M.C.; Buonsenso, D.; Ferro, V.; Valentini, P.; Magistrelli, A.; Lombardi, M.H.; Romani, L.; D’Argenio, P.; Campana, A.; et al. Roman Lung Ultrasound Study Team for Pediatric COVID-19 (ROMULUS COVID Team). Lung Ultrasound in Children with COVID-19: Preliminary Findings. Ultrasound Med. Biol. 2020, 46, 2094–2098. [Google Scholar] [CrossRef]
- Agostinis, P.; Copetti, R.; Lapini, L.; Monteiro, G.B.; N’Deque, A.; Baritussio, A. Chest ultrasound findings in pulmonary tuberculosis. Trop. Dr. 2017, 47, 320–328. [Google Scholar] [CrossRef]
- Hunter, L.; Bélard, S.; Janssen, S.; van Hoving, D.J.; Heller, T. Miliary tuberculosis: Sonographic pattern in chest ultrasound. Infection 2016, 44, 243–246. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.M.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic Properties of Chest Ultrasound in Thoracic Tuberculosis—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2235. [Google Scholar] [CrossRef]
- Montuori, M.; Casella, F.; Casazza, G.; Franzetti, F.; Pini, P.; Invernizzi, C.; Torzillo, D.; Rizzardini, G.; Galli, M.; Cogliati, C. Lung ultrasonography in pulmonary tuberculosis: A pilot study on diagnostic accuracy in a high-risk population. Eur. J. Intern. Med. 2019, 66, 29–34. [Google Scholar] [CrossRef]
- Buonsenso, D.; Curatola, A.; Valentini, P.; Scialanga, B.; Toma, P.; Musolino, A.M. Chest ultrasound findings in children with confirmed pulmonary tuberculosis in low tuberculosis incidence country. Pediatr. Pulmonol. 2019, 54, 1348–1350. [Google Scholar] [CrossRef]
- Fentress, M.; Ugarte-Gil, C.; Cervantes, M.; Rivas, D.; Moore, D.; Caliguiri, P.; Bergman, K.; Noazin, S.; Padovani, A.; Gilman, R.H. Lung Ultrasound Findings Compared with Chest X-Ray Findings in Known Pulmonary Tuberculosis Patients: A Cross-Sectional Study in Lima, Peru. Am. J. Trop. Med. Hyg. 2020, 103, 1827–1833. [Google Scholar] [CrossRef]
- Heuvelings, C.C.; Bélard, S.; Andronikou, S.; Jamieson-Luff, N.; Grobusch, M.P.; Zar, H.J. Chest ultrasound findings in children with suspected pulmonary tuberculosis. Pediatr. Pulmonol. 2019, 54, 463–470. [Google Scholar] [CrossRef]
- Heuvelings, C.C.; Bélard, S.; Andronikou, S.; Lederman, H.; Moodley, H.; Grobusch, M.P.; Zar, H.J. Chest ultrasound compared to chest X-ray for pediatric pulmonary tuberculosis. Pediatr. Pulmonol. 2019, 54, 1914–1920. [Google Scholar] [CrossRef]
- Rea, G.; Sperandeo, M.; Lieto, R.; Bocchino, M.; Quarato, C.M.I.; Feragalli, B.; Valente, T.; Scioscia, G.; Giuffreda, E.; Barbaro, M.P.F.; et al. Chest Imaging in the Diagnosis and Management of Pulmonary Tuberculosis: The Complementary Role of Thoraci Ultrasound. Front. Med. 2021, 8, 753821. [Google Scholar] [CrossRef]
- Bigio, J.; Kohli, M.; Klinton, J.S.; MacLean, E.; Gore, G.; Small, P.M.; Ruhwald, M.; Weber, S.F.; Jha, S.; Pai, M. Diagnostic accuracy of point-of-care ultrasound for pulmonary tuberculosis: A systematic review. PLoS ONE 2021, 16, e0251236. [Google Scholar] [CrossRef]
- Suttels, V.; Du Toit, J.D.; Fiogbé, A.A.; Wachinou, A.P.; Guendehou, B.; Alovokpinhou, F.; Toukoui, P.; Hada, A.R.; Sefou, F.; Vinasse, P.; et al. Point-of-care ultrasound for tuberculosis management in Sub-Saharan Africa—A balanced SWOT analysis. Int. J. Infect. Dis. 2022, 123, 46–51. [Google Scholar] [CrossRef]
- Abrokwa, S.K.; Ruby, L.C.; Heuvelings, C.C.; Bélard, S. Task shifting for point of care ultrasound in primary healthcare in low- and middle-income countries—A systematic review. eClinicalMedicine 2022, 45, 101333. [Google Scholar] [CrossRef]
| UTILITY | LIMITS |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morello, R.; De Rose, C.; Ferrari, V.; Valentini, P.; Musolino, A.M.; Biasucci, D.G.; Vetrugno, L.; Buonsenso, D. Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review. J. Clin. Med. 2022, 11, 5714. https://doi.org/10.3390/jcm11195714
Morello R, De Rose C, Ferrari V, Valentini P, Musolino AM, Biasucci DG, Vetrugno L, Buonsenso D. Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review. Journal of Clinical Medicine. 2022; 11(19):5714. https://doi.org/10.3390/jcm11195714
Chicago/Turabian StyleMorello, Rosa, Cristina De Rose, Vittoria Ferrari, Piero Valentini, Anna Maria Musolino, Daniele Guerino Biasucci, Luigi Vetrugno, and Danilo Buonsenso. 2022. "Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review" Journal of Clinical Medicine 11, no. 19: 5714. https://doi.org/10.3390/jcm11195714
APA StyleMorello, R., De Rose, C., Ferrari, V., Valentini, P., Musolino, A. M., Biasucci, D. G., Vetrugno, L., & Buonsenso, D. (2022). Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review. Journal of Clinical Medicine, 11(19), 5714. https://doi.org/10.3390/jcm11195714








