Addressing Drug Resistance in Cancer: A Team Medicine Approach
Abstract
1. Introduction
2. Is Drug Resistance Genetic or, Are Non-Genetic Mechanisms Involved?
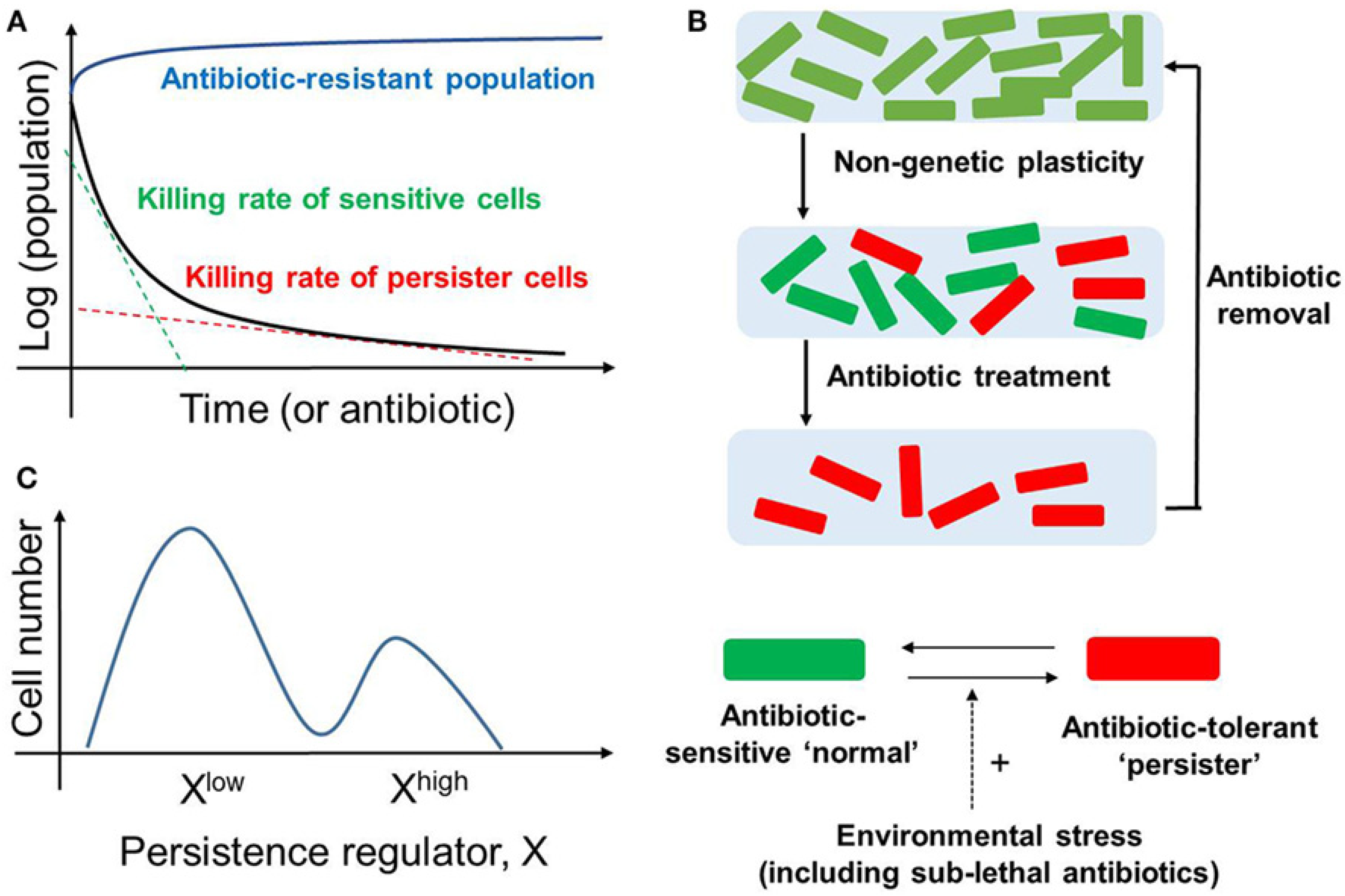
3. Discerning Drug Tolerance and Resistance
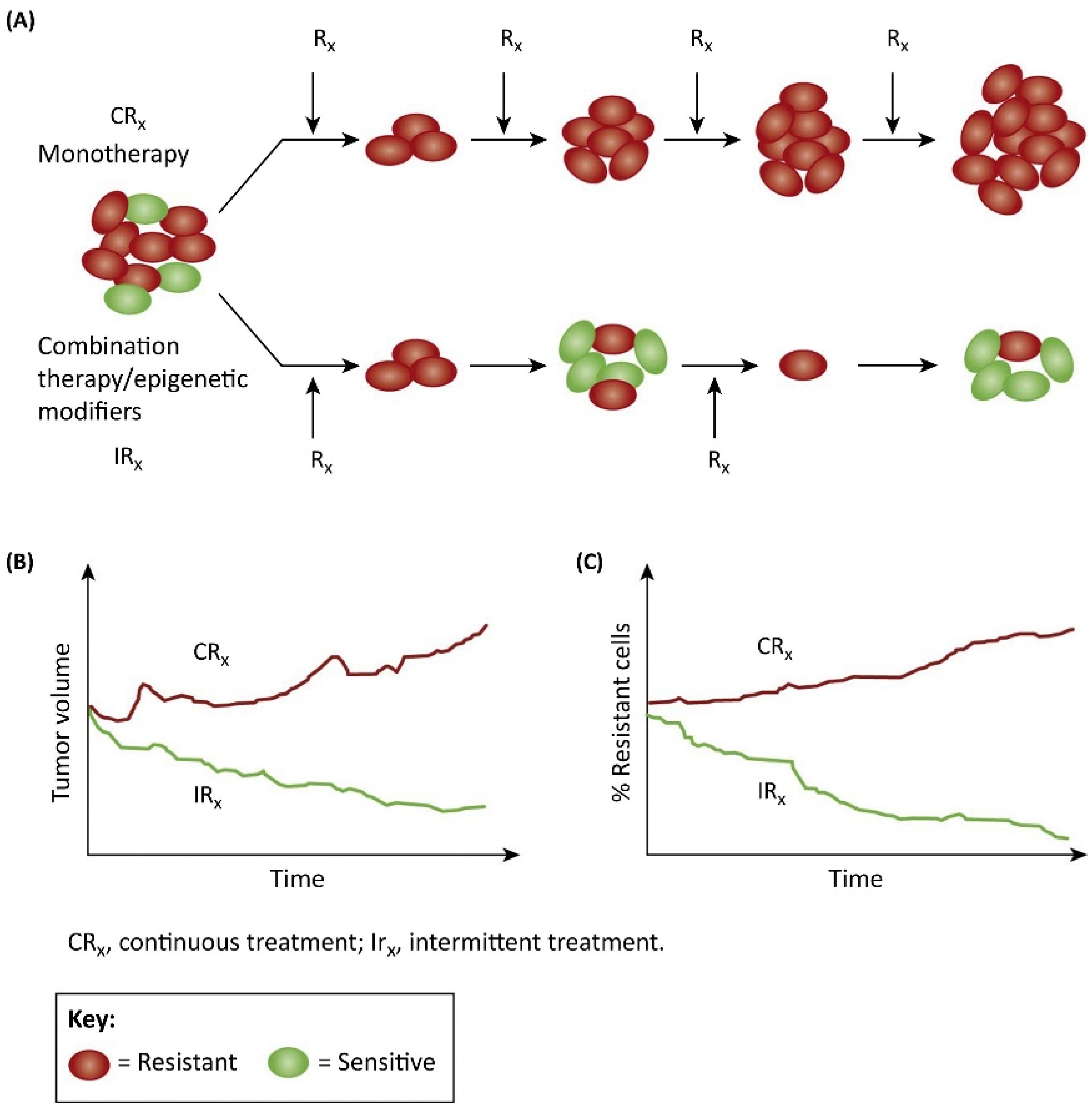
4. Current Treatment Strategies May Be Counterproductive
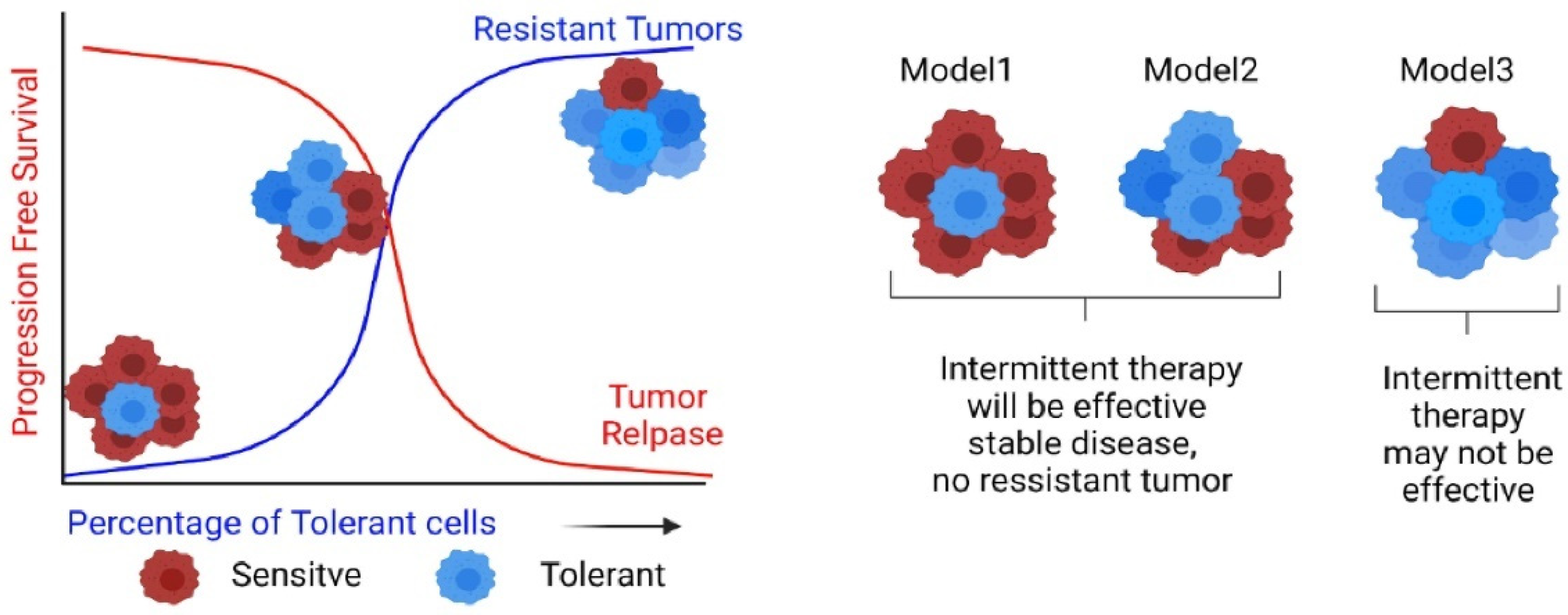
5. Emergence of Irreversible Drug Resistance via a Potentially Reversible Tolerant State
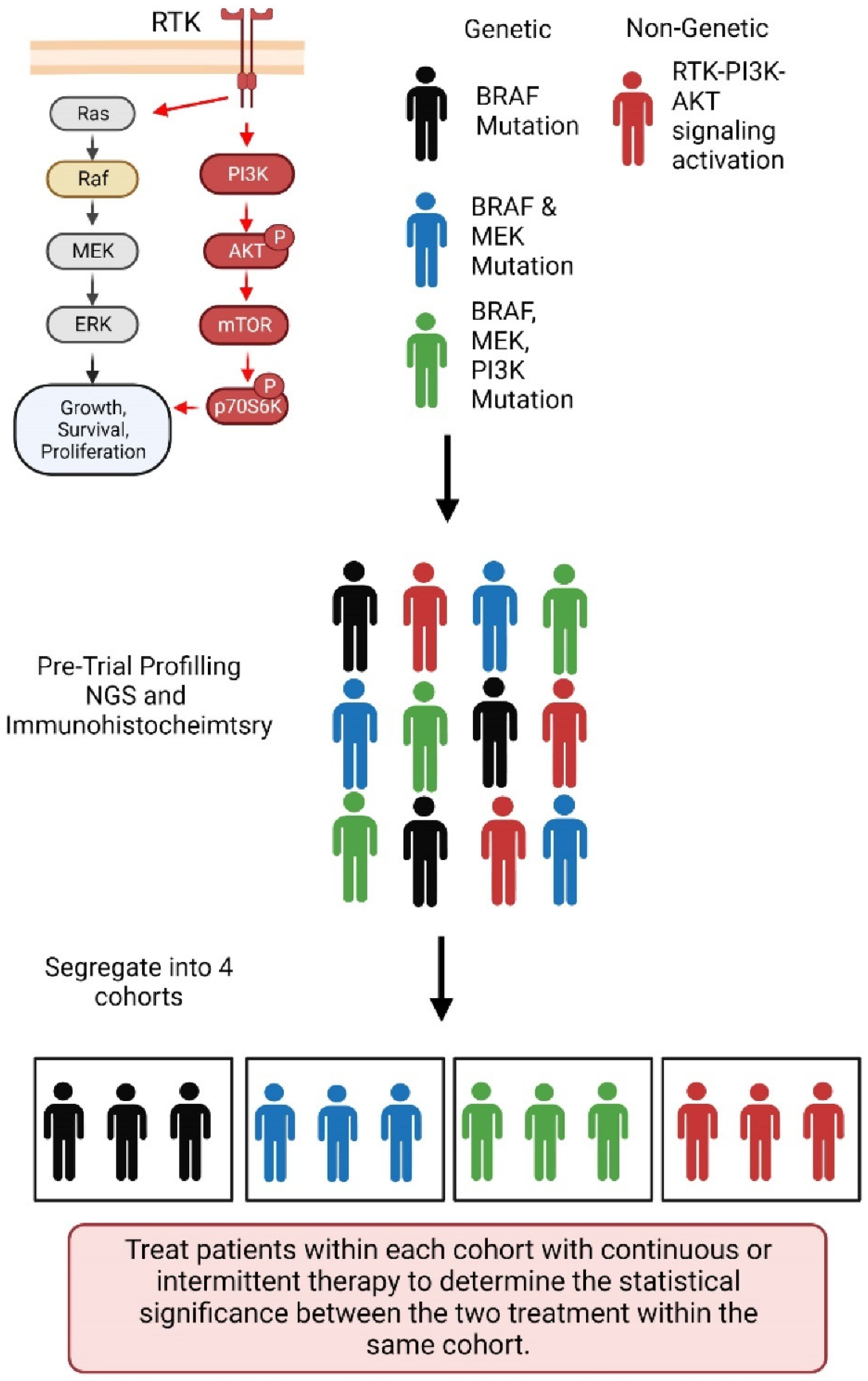
6. Intermittent or ‘Adaptive’ Therapy—An Eco-Evolutionary Principles-Based Therapeutic Strategy to Preclude or Delay Onset of Drug Resistance
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cancer.org/research/cancer-facts-statistics/global.html (accessed on 1 September 2022).
- Salgia, R.; Kulkarni, P. Integrating Clinical and Translational Research Networks—Building Team Medicine. J. Clin. Med. 2020, 9, 2975. [Google Scholar] [CrossRef] [PubMed]
- Hansford, S.; Huntsman, D.G. Boveri at 100: Theodor Boveri and genetic predisposition to cancer. J. Pathol. 2014, 234, 142–145. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A. Marcella O’Grady Boveri (1865–1950) and the chromosome theory of cancer. J. Med. Genet. 1985, 6, 431–440. [Google Scholar] [CrossRef]
- Boveri, T. Zur Frage der Entstehung Maligner Tumoren; Verlag von Gustav Fischer: Jena, Germany, 1914. [Google Scholar]
- Boveri, T. The Origin of Mnalignzatnt Tunors. (Translated by M Boveri.); Williams and Wilkins: Baltimore, MD, USA, 1929. [Google Scholar]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The Path to Cancer—Three Strikes and You’re Out. N. Engl. J. Med. 2015, 373, 1895–1898. [Google Scholar] [CrossRef]
- Laland, K.; Uller, T.; Feldman, M.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J.; Wray, G.A.; Hoekstra, H.E.; et al. Does evolutionary theory need a rethink? Nature 2014, 514, 161–164. [Google Scholar] [CrossRef]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting mutations in cancer. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Salgia, R.; Pharaon, R.; Mambetsariev, I.; Nam, A.; Sattler, M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep. Med. 2021, 2, 100186. [Google Scholar] [CrossRef]
- Pagliarini, R.; Shao, W.; Sellers, W.R. Oncogene addiction: Pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015, 16, 280–296. [Google Scholar] [CrossRef]
- Bell, C.C.; Gilan, O. Principles and mechanisms of non-genetic resistance in cancer. Br. J. Cancer 2020, 122, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Shlyakhtina, Y.; Moran, K.; Portal, M. Genetic and Non-Genetic Mechanisms Underlying Cancer Evolution. Cancers 2021, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Achuthan, S.; Bhattacharya, S.; Jolly, M.K.; Kotnala, S.; Leite, V.B.P.; Mohanty, A.; Orban, J.; Roy, S.; Rangarajan, G.; et al. Protein conformational dynamics and phenotypic switching. Biophys. Rev. 2021, 13, 1127–1138. [Google Scholar] [CrossRef]
- Kulkarni, V.; Kulkarni, P. Intrinsically disordered proteins and phenotypic switching: Implications in cancer. Prog. Mol. Biol. Transl. Sci. 2019, 166, 63–84. [Google Scholar] [CrossRef]
- Bowler, E.H.; Wang, Z.; Ewing, R.M. How do oncoprotein mutations rewire protein-protein interaction networks? Expert Rev. Proteom. 2015, 12, 449–455. [Google Scholar] [CrossRef][Green Version]
- Paliouras, M.; Zaman, N.; Lumbroso, R.; Kapogeorgakis, L.; Beitel, L.K.; Wang, E.; Trifiro, M. Dynamic rewiring of the androgen receptor protein interaction network correlates with prostate cancer clinical outcomes. Integr. Biol. 2011, 3, 1020–1032. [Google Scholar] [CrossRef]
- Salgia, R.; Kulkarni, P. The Genetic/Non-genetic Duality of Drug ‘Resistance’ in Cancer. Trends Cancer 2018, 4, 110–118. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Gupta, S.; Singh, H.P.; Kumar, S.; Gautam, A.; Kapoor, P.; Raghava, G.P.S. Cancer DR: Cancer drug resistance database. Sci. Rep. 2013, 3, srep01445. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Kulkarni, P.; Weninger, K.; Orban, J.; Levine, H. Phenotypic Plasticity, Bet-Hedging, and Androgen Independence in Prostate Cancer: Role of Non-Genetic Heterogeneity. Front. Oncol. 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2021, 11, 599965. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.; Tsyganov, M.; Litviakov, N. Tumour Stem Cells in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 5058. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Huang, S. Reconciling Non-Genetic Plasticity with Somatic Evolution in Cancer. Trends Cancer 2021, 7, 309–322. [Google Scholar] [CrossRef]
- Gomez, K.; Rabadan, R. A persistent look at how tumours evade therapy. Nature 2021, 596, 491–493. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mohanty, A.; Achuthan, S.; Kotnala, S.; Jolly, M.K.; Kulkarni, P.; Salgia, R. Group Behavior and Emergence of Cancer Drug Resistance. Trends Cancer 2021, 7, 323–334. [Google Scholar] [CrossRef]
- Ramirez, M.; Rajaram, S.; Steininger, R.J.; Osipchuk, D.; Roth, M.A.; Morinishi, L.S.; Evans, L.; Ji, W.; Hsu, C.-H.; Thurley, K.; et al. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat. Commun. 2016, 7, 10690. [Google Scholar] [CrossRef]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hütter, J.-C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021, 596, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Strategy of the Genes; Geo Allen & Unwin: London, UK, 1957. [Google Scholar]
- Mahmoudabadi, G.; Rajagopalan, K.; Getzenberg, R.H.; Hannenhalli, S.; Rangarajan, G.; Kulkarni, P. Intrinsically disordered proteins and conformational noise: Implications in cancer. Cell Cycle 2013, 12, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Kessler, D.A.; Levine, H. Biological Networks Regulating Cell Fate Choice are Minimally Frustrated. Phys. Rev. Lett. 2020, 125, 088101. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.G.; Schafer, N.P.; Radusky, L.G.; Tsai, M.-Y.; Guzovsky, A.B.; Wolynes, P.G.; Ferreiro, D.U. Protein Frustratometer 2: A tool to localize energetic frustration in protein molecules, now with electrostatics. Nucleic Acids Res. 2016, 44, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P. Intrinsically Disordered Proteins: Insights from Poincaré, Waddington, and Lamarck. Biomolecules 2020, 10, 1490. [Google Scholar] [CrossRef]
- Kulkarni, P.; Leite, V.B.P.; Roy, S.; Bhattacharyya, S.; Mohanty, A.; Achuthan, S.; Singh, D.; Appadurai, R.; Rangarajan, G.; Weninger, K.; et al. Intrinsically disordered proteins: Ensembles at the limits of Anfinsen’s dogma. Biophys. Rev. 2022, 3, 011306. [Google Scholar] [CrossRef]
- Liu, Z.; Miller, D.; Li, F.; Liu, X.; Levy, S.F. A large accessory protein interactome is rewired across environments. Elife 2020, 9, e62365. [Google Scholar] [CrossRef]
- Huang, S.; Ernberg, I.; Kauffman, S. Cancer attractors: A systems view of tumors from a gene network dynamics and developmental perspective. Semin. Cell Dev. Biol. 2009, 20, 869–876. [Google Scholar] [CrossRef]
- Li, Q.; Wennborg, A.; Aurell, E.; Dekel, E.; Zou, J.-Z.; Xu, Y.; Huang, S.; Ernberg, I. Dynamics inside the cancer cell attractor reveal cell heterogeneity, limits of stability, and escape. Proc. Natl. Acad. Sci. USA 2016, 113, 2672–2677. [Google Scholar] [CrossRef]
- Fitzgerald, D.M.; Hastings, P.; Rosenberg, S.M. Stress-Induced Mutagenesis: Implications in Cancer and Drug Resistance. Annu. Rev. Cancer Biol. 2017, 1, 119–140. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Brown, J.S. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb Perspect. Med. 2020, 10, a040972. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Luddy, K.A.; Robertson-Tessi, M.; O’Farrelly, C.; Gatenby, R.A.; Anderson, A.R. Searching for Goldilocks: How Evolution and Ecology Can Help Uncover More Effective Patient-Specific Chemotherapies. Cancer Res. 2020, 80, 5147–5154. [Google Scholar] [CrossRef] [PubMed]
- Kavran, A.J.; Stuart, S.A.; Hayashi, K.R.; Basken, J.M.; Brandhuber, B.J.; Ahn, N.G. Intermittent treatment of BRAFV600E melanoma cells delays resistance by adaptive resensitization to drug rechallenge. Proc. Natl. Acad. Sci. USA 2022, 119, e2113535119. [Google Scholar] [CrossRef]
- Felder, S.I.; Fleming, J.B.; Gatenby, R.A. Treatment-induced evolutionary dynamics in nonmetastatic locally advanced rectal adenocarcinoma. Adv. Cancer Res. 2021, 151, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Metts, J.; Pressley, M.; Fridley, B.L.; Hayashi, M.; Isakoff, M.S.; Loeb, D.M.; Makanji, R.; Roberts, R.D.; Trucco, M.; et al. An evolutionary framework for treating pediatric sarcomas. Cancer 2020, 126, 2577–2587. [Google Scholar] [CrossRef]
- Nam, A.; Mohanty, A.; Bhattacharya, S.; Kotnala, S.; Achuthan, S.; Hari, K.; Srivastava, S.; Guo, L.; Nathan, A.; Chatterjee, R.; et al. Dynamic Phenotypic Switching and Group Behavior Help Non-Small Cell Lung Cancer Cells Evade Chemotherapy. Biomolecules 2021, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Dunnett-Kane, V.; Nicola, P.; Blackhall, F.; Lindsay, C. Mechanisms of Resistance to KRASG12C Inhibitors. Cancers 2021, 13, 151. [Google Scholar] [CrossRef]
- Tanaka, N.; Lin, J.J.; Li, C.; Ryan, M.B.; Zhang, J.; Kiedrowski, L.A.; Michel, A.G.; Syed, M.U.; Fella, K.A.; Sakhi, M.; et al. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov. 2021, 11, 1913–1922. [Google Scholar] [CrossRef]
- Koga, T.; Suda, K.; Fujino, T.; Ohara, S.; Hamada, A.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Arita, T.; et al. KRAS Secondary Mutations That Confer Acquired Resistance to KRAS G12C Inhibitors, Sotorasib and Adagrasib, and Overcoming Strategies: Insights From the In Vitro Experiments. J. Thorac. Oncol. 2021, 16, 1321–1332. [Google Scholar] [CrossRef]
- Addeo, A.; Banna, G.; Friedlaender, A. KRAS G12C Mutations in NSCLC: From Target to Resistance. Cancers 2021, 13, 2541. [Google Scholar] [CrossRef]
- Reck, M.; Carbone, D.; Garassino, M.; Barlesi, F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Zhao, Y.; Aronowitz, J.; Mai, T.T.; Vides, A.; Qeriqi, B.; Kim, D.; Li, C.; de Stanchina, E.; Mazutis, L.; et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature 2020, 577, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Navas, P.M.; Wojtkowiak, J.W.; Gatenby, R.A. Application of Evolutionary Principles to Cancer Therapy. Cancer Res. 2015, 75, 4675–4680. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Martelotto, L.; Baslan, T.; Vides, A.; Solomon, M.; Mai, T.T.; Chaudhary, N.; Riely, G.J.; Li, B.T.; Scott, K.; et al. An approach to suppress the evolution of resistance in BRAFV600E-mutant cancer. Nat. Med. 2017, 23, 929–937. [Google Scholar] [CrossRef]
- Gonzalez-Cao, M.; Casas, C.M.D.L.; Oramas, J.; Berciano-Guerrero, M.A.; de la Cruz, L.; Cerezuela, P.; Arance, A.; Muñoz-Couselo, E.; Espinosa, E.; Puertolas, T.; et al. Intermittent BRAF inhibition in advanced BRAF mutated melanoma results of a phase II randomized trial. Nat. Commun. 2021, 12, 7008. [Google Scholar] [CrossRef]
- Algazi, A.P.; Othus, M.; Daud, A.I.; Lo, R.S.; Mehnert, J.M.; Truong, T.-G.; Conry, R.; Kendra, K.; Doolittle, G.C.; Clark, J.I.; et al. Continuous versus intermittent BRAF and MEK inhibition in patients with BRAF-mutated melanoma: A randomized phase 2 trial. Nat. Med. 2020, 26, 1564–1568. [Google Scholar] [CrossRef]
- Maio, M.; Carlino, M.S.; Joshua, A.M.; McWhirter, E.; Ribas, A.; Ascierto, P.A.; Miller, W.H., Jr.; Butler, M.O.; Ferrucci, P.F.; Zielinski, R.R.; et al. KEYNOTE-022: Pembrolizumab with trametinib in patients with BRAF wild-type melanoma or advanced solid tumours irrespective of BRAF mutation. Eur. J. Cancer 2022, 160, 1–11. [Google Scholar] [CrossRef]
- Hussain, M.; Tangen, C.M.; Berry, D.L.; Higano, C.S.; Crawford, E.D.; Liu, G.; Wilding, G.; Prescott, S.; Sundaram, S.K.; Small, E.J.; et al. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer. N. Engl. J. Med. 2013, 368, 1314–1325. [Google Scholar] [CrossRef]
- Crook, J.M.; O’Callaghan, C.J.; Duncan, G.; Dearnaley, D.P.; Higano, C.S.; Horwitz, E.M.; Frymire, E.; Malone, S.; Chin, J.; Nabid, A.; et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med. 2012, 367, 895–903. [Google Scholar] [CrossRef]
- Schulman, C.; Cornel, E.; Matveev, V.; Tammela, T.L.; Schraml, J.; Bensadoun, H.; Warnack, W.; Persad, R.; Salagierski, M.; Veiga, F.G.; et al. Intermittent Versus Continuous Androgen Deprivation Therapy in Patients with Relapsing or Locally Advanced Prostate Cancer: A Phase 3b Randomised Study (ICELAND). Eur. Urol. 2016, 69, 720–727. [Google Scholar] [CrossRef]
- Tsai, H.-T.; Pfeiffer, R.M.; Philips, G.K.; Barac, A.; Fu, A.Z.; Penson, D.; Zhou, Y.; Potosky, A.L. Risks of Serious Toxicities from Intermittent versus Continuous Androgen Deprivation Therapy for Advanced Prostate Cancer: A Population Based Study. J. Urol. 2017, 197, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Roberts, M.J.; Klotz, L.; Higano, C.S.; Papa, N.; Sengupta, S.; Bolton, D.; Lawrentschuk, N. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat. Rev. Urol. 2020, 17, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.T.; Burkett, J.M.; Nelson, R.S.; Pow-Sang, J.M.; Gatenby, R.A.; Kubal, T.; Peabody, J.W.; Letson, G.D.; McLeod, H.L.; Zhang, J. Budget Impact of Adaptive Abiraterone Therapy for Castration-Resistant Prostate Cancer. Am. Health Drug Benefits 2021, 14, 15–20. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, P.; Mohanty, A.; Bhattacharya, S.; Singhal, S.; Guo, L.; Ramisetty, S.; Mirzapoiazova, T.; Mambetsariev, B.; Mittan, S.; Malhotra, J.; et al. Addressing Drug Resistance in Cancer: A Team Medicine Approach. J. Clin. Med. 2022, 11, 5701. https://doi.org/10.3390/jcm11195701
Kulkarni P, Mohanty A, Bhattacharya S, Singhal S, Guo L, Ramisetty S, Mirzapoiazova T, Mambetsariev B, Mittan S, Malhotra J, et al. Addressing Drug Resistance in Cancer: A Team Medicine Approach. Journal of Clinical Medicine. 2022; 11(19):5701. https://doi.org/10.3390/jcm11195701
Chicago/Turabian StyleKulkarni, Prakash, Atish Mohanty, Supriyo Bhattacharya, Sharad Singhal, Linlin Guo, Sravani Ramisetty, Tamara Mirzapoiazova, Bolot Mambetsariev, Sandeep Mittan, Jyoti Malhotra, and et al. 2022. "Addressing Drug Resistance in Cancer: A Team Medicine Approach" Journal of Clinical Medicine 11, no. 19: 5701. https://doi.org/10.3390/jcm11195701
APA StyleKulkarni, P., Mohanty, A., Bhattacharya, S., Singhal, S., Guo, L., Ramisetty, S., Mirzapoiazova, T., Mambetsariev, B., Mittan, S., Malhotra, J., Gupta, N., Kim, P., Babikian, R., Rajurkar, S., Subbiah, S., Tan, T., Nguyen, D., Merla, A., Kollimuttathuillam, S. V., ... Salgia, R. (2022). Addressing Drug Resistance in Cancer: A Team Medicine Approach. Journal of Clinical Medicine, 11(19), 5701. https://doi.org/10.3390/jcm11195701







