1. Introduction
The incidence of differentiated thyroid cancer is rapidly increasing around the globe and, with a crude prevalence of 11% in the United Arab Emirates (UAE), became the second most common type of cancer in women in the UAE. As a consequence of this so-called “thyroid cancer epidemic,” the thyroidectomy rate is increasing, which, in turn, is a major cause of unilateral vocal fold paralysis (UVFP). Currently, the percentage of postoperative vocal fold nerve injuries is estimated to be between 1% and 30% for transient lesions and between 0.5% and 5% for permanent ones. UVFP is responsible for disabling dysphonia, low quality of life, and a range of medico-legal implications [
1].
A non-recurrent laryngeal nerve (non-RLN) is a rare and more complex anatomical variant of the recurrent laryngeal nerve (RLN) [
2,
3]. It has an aberrant pathway that directly enters the larynx without first descending into the thorax [
4]. It is mainly found on the right side of the body, with an incidence rate of 0.5–1%, whereas the left-sided non-RLN is extremely rare [
5]. The presence of a non-RLN makes a person prone to injury during thyroidectomy and leads to severe postoperative complications if not treated carefully. It has been reported previously that the occurrence of postoperative nerve injury increases by six times if a non-RLN remains undetected [
6].
Intraoperative neuromonitoring has been gaining popularity as a supplement to visual identification, due to its usefulness in detecting RLN during thyroid and parathyroid surgery and preventing injury [
7]. However, when the surgeon cannot detect an RLN, he must consider that a non-RLN is present [
8]. Two types of non-RLN can be identified—type 1 and type 2 [
9,
10]. A type-1 non-RLN travels along the superior thyroid pedicle, while type 2 follows the path of the inferior thyroid artery [
8]. During thyroidectomy, both types must be identified; the best method to detect them is via intraoperative neuromonitoring [
8]. A neck CT scan or MRI can identify the vessel’s position. However, this raises the cost of the operation significantly and also incorporates a high radiation dose [
8]. Intraoperative neuromonitoring can be a suitable alternative in this regard. It is associated with a higher identification rate of non-RLN than any other method, at almost 6% [
8].
The proper identification of non-RLN can be followed by a successful thyroidectomy where nerve functionality is restored after the surgery. Using intraoperative neuromonitoring, surgeons can retrace their surgical path so that no injury befalls a nerve [
11]. Therefore, this study is the first to describe the clinical use of the NIM Vital
TM neuromonitoring system (Medtronic Xomed, Inc., Jacksonville, FL, USA). Furthermore, the ADNM technique was performed at precisely defined anatomical positions in a large patient cohort without the use of a neuromuscular blockade for endotracheal intubation, which enabled the easy identification of right-sided non-RLN during thyroidectomy and the prevention of voice dysfunction after surgery. The narrow operative field of a minimally invasive open thyroidectomy and the absence of neuromuscular blockade restrict the degree to which surgical instruments can be moved. These kinds of facts drive surgeons to create more novel surgical procedures in order to limit the risk of vocal nerve injury that is associated with serious voice disorders.
The small operational field in a minimally invasive open thyroidectomy, as performed, and not using any neuromuscular blockade limiting instrument-maneuvering degree offers surgeons the opportunity for more innovative surgical techniques that can avoid any neural injury and prevent major voice disorders.
3. Results and Discussion
Overall, 170 patients were enrolled in this study, with a total of 270 nerves at risk. In total, 136 women with a mean age of 40 years (range 18–74) and 36 men with a mean age of 42 (range 21–66), with 270 nerves at risk, were included in the analysis. Indications for surgery were malignancy in 70 patients, toxic goiter/Graves in 23 patients, retrosternal goiter in 21 patients, and symptomatic multinodular goiter in 64 cases. Of these, 100 patients received a total thyroidectomy, 46 patients had a right lobectomy, and 24 had a left lobectomy only. For the total thyroidectomy, the median skin-to-skin surgery duration was 52 min (range 24–104 min) and the median hospital stay was 2 days (range 1–4 days). In four cases (4/146; 2.74%), a non-RLN was discovered. All patients with a non-RLN were females aged 33–45 years.
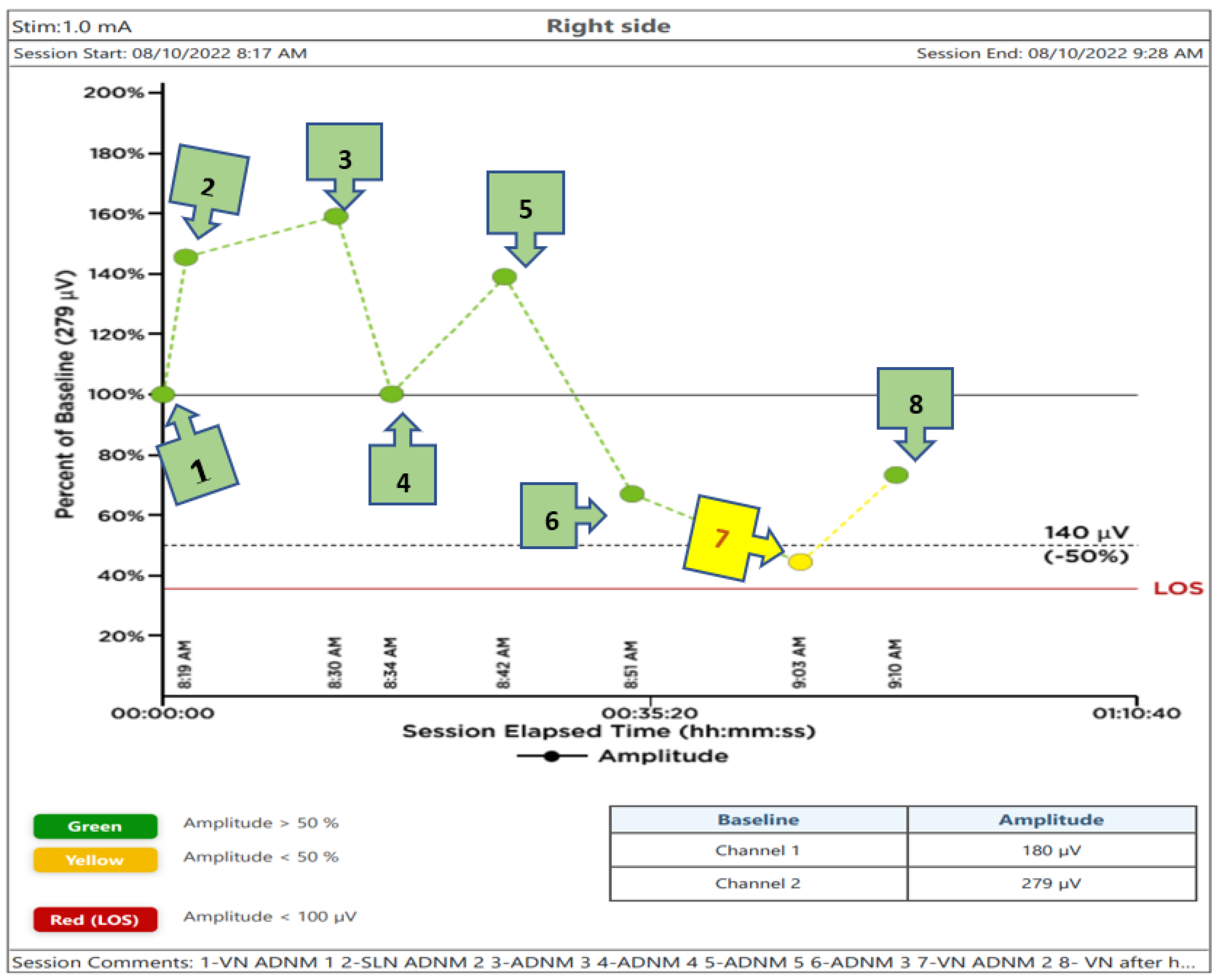
A diagram showing the recorded voltage values of the subsequent steps of the performed thyroidectomy is displayed in
Figure 6. In this figure, the signal from the VN before dissection and below the (ITA) was chosen as the 100% baseline value. Recorded voltages below or above it, taken at different points as mentioned above, are also plotted in the Figure.
The positive signal of ADNM-VN1 confirmed the presence of a right-sided non-RLN. A similar procedure was also conducted on the left side and no such signal could be obtained. This proves that the identification of non-RLN, conducted before continuing with the dissection, was successful. As the dissection went on, the nerves continued to generate EMG values, which are plotted in
Figure 6. Non-RLN type 1 and type 2 were subsequently identified (
Figure 3,
Figure 4 and
Figure 5) and their presence was confirmed by the positive EMG values. However, the amplitude of the EMG signal of the VN dropped below 50% of the baseline when it was recorded post-dissection. Since the data was recorded immediately after the dissection was over, the VN was almost numb and did not recover immediately. Therefore, the non-RLN could not generate any EMG data. After about 7 min and treatment with hydrocortisone, the VN started to recover, and an EMG signal could be recorded. This proves that the minimally invasive surgery was successful after using the ADNM protocol of stimulating precisely defined anatomical points; because of this, there was no nerve injury. The non-RLN was completely fine, and its function was intact.
This study is the first of its kind to utilize the NIM Vital
TM device to report the outcome in this large cohort, wherein the EMG signal of different anatomical points was measured. Neuromuscular blockade agents are typically used in anesthesia for endotracheal intubation. However, this may lead to the potentiation of these blockades, which leads to postoperative complications [
12]. Therefore, in this study, no neuromuscular blocking agents were used; nonetheless, the thyroidectomy was successful. We were also able to identify the precise anatomical points where stimulation should be applied to identify the non-RLN without damaging its functionality, meaning that this is the first study in this field. As the prevalence of non-RLN is higher than previously expected, a thyroidectomy can prove to be difficult [
3]. Our ADNM protocol for mapping the anatomical points can provide a guide for the surgeons so that a minimally invasive thyroidectomy can be conducted without the loss of function of non-RLN. However, our developed protocol can also be applied if the presence of non-RLN is not apparent. In other words, this protocol can be used with equal effectiveness in standard thyroidectomy procedures, where the surgeons will follow the anatomical points that have been precisely identified in the above protocol. Another important feature is that the thyroidectomy can be performed without using any neuromuscular blockade, to minimize the confounder with the EMG signal and its amplitude, and to save costs by avoiding muscle relaxants and antidotes and optimizing OT utilization, particularly in saving the time from the Kocher incision until the complete lobe resection, which varies, according to the surgeon, between 10 and 30 min, with an average of 18 min. The second point to minimize confounders or indirect damage to the VN or RLN is in postponing the use of energy or electrical sealing devices, as well as visualization and traction of the thyroid lobe, to reduce any possible risk of impaired EMG-amplitude caused by heat, electrical field, or mechanical traction. Those two steps will ensure the achievement of the real baseline EMG-amplitude. The use of an energy-based device can result in the paralysis of RLN, which can cause hoarseness after the operation [
13]. The precise definition of those stimulation points is of great importance for both novices in thyroid surgery and new users of intraoperative neuromonitoring. The colored EMG value will allow the surgeon to constantly monitor his steps and correct his missteps in the case of any red signal points during the procedure. This correction can be made either by using less traction on the luxated lobe, by the revision of nerve entrapment in clips or sutures, preventing heat spread from vessel-sealing devices, or by tube correction, and changing the current settings on the NIM Vital
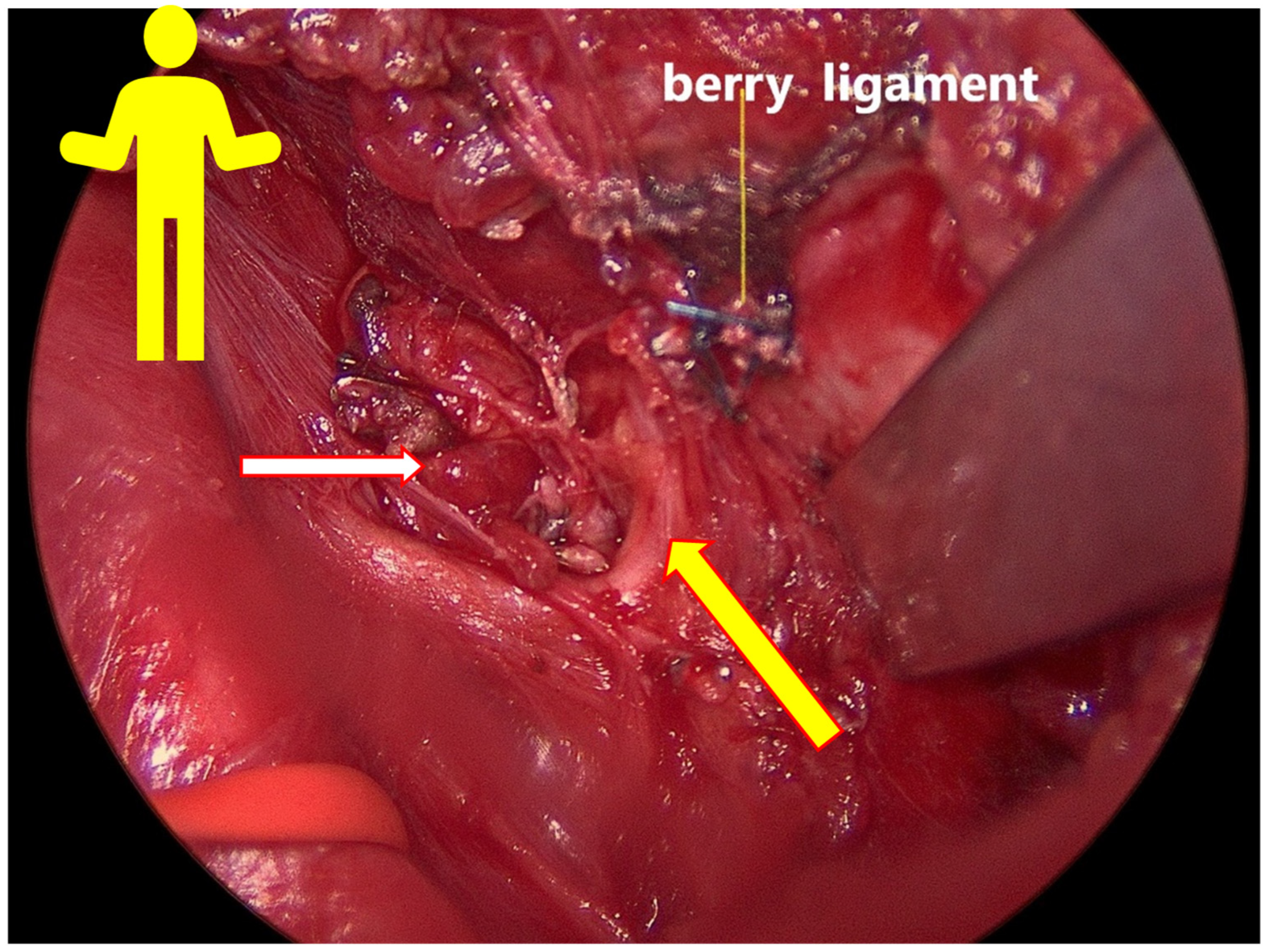
TM device. Furthermore, through the use of the ADNM protocol, a retrosternal goiter can be resected much more safely, using the ADNM mappings points before any forced mobilization of the retrosternal goiter.
This methodology includes several novel and important steps during thyroidectomy that will increase the chance of preventing voice dysfunction in the RLN, SLN, and non-RLN. For example, to protect the SLN, the thyroid gland was clamped very close to the upper pole vessels, being lifted from the prevertebral facia both distally and laterally. A vascular plane between the larynx muscle and upper pole vessels is of great importance when locating the SLN and protecting it from mechanical or thermal damage. Here, intermittent INOM testing for every portion that is planned for division is mandatory. The ligation should be performed after a clear capsule is closed, separating it into 2–3 lengths. The traction directions of the strap muscles and thyroid during surgery helped in the visualization of the nerves. Intraoperative thermal injury can occur due to the use of electricity or energy-based devices [
14]. The energy-based devices were used with significant precautions. Damage to the SLN and non-RLN due to heat spread was prevented by cooling the area with cold saline after every heating device was used, in a radius of 2 cm to the nerve; otherwise, clips or 4.0 PDS stitches were used. The saline took away the excess heat and protected the nerves. Finally, by a sudden decrease in EMG-amplitude (point 7 in
Figure 6) an intravenous injection of 200 mg hydrocortisone with 10–15 min of cooling of the field and the removal of all traction trocars on the situs can help to recover EMG-amplitude and continue the procedure in a relaxed OT-environment. Prior research has shown that individuals with temporary RLN-paresis recover faster corticosteroids are delivered intraoperatively, but the same research has failed to establish that a single dose of intraoperative corticosteroids can reduce the rate of temporary/permanent RLN- paresis after surgery [
15,
16].
Our study is limited by its retrospective nature and its relatively small patient sample. Controlled, randomized studies with larger patient samples are needed to compare the ADNM technique to the gold standard of thyroidectomy. The low and uncertain prevalence of non-RLN is another obstacle.
4. Conclusions
To our knowledge, this is the first study describing the clinical benefit of using the new NIM Vital™ (Medtronic Xomed, Inc., Jacksonville, FL, USA) in a large patient cohort, without the use of any neuromuscular blockade for intubation.
The developed ADNM protocol was successful in stimulating precisely defined anatomical points on the VN and SLN to identify the non-RLN accurately during a minimally invasive thyroidectomy. These stimulation points helped guide the surgeon for the early detection of non-RLN. Obtained EMG signal values were compared with the baseline value of VN pre-dissection, which was taken as 100%. The post-dissection values of SLN and VN fell well below the baseline, to the point where the nerves were considered numb. However, after treatment with hydrocortisone, the VN recovered, and voice functionality was intact. During the surgery, besides the specified anatomical points, novel steps were taken that increased the chance of the visualization of nerves and preservation of their functions, without using any endoscopic optical instruments, such as the safe ligation of upper pole vessels, cooling of the same area to prevent thermal injury, specified traction direction, and hydrocortisone application for quick recovery of the nerves. Our developed ADNM protocol has the potential to improve cost-effectiveness by better OT utilization through fast and secure surgery, reducing expensive muscle relaxants and antidotes. Even without the presence of non-RLN, the same principles should be applied. Endocrine surgeons can monitor their surgical procedures in real time by observing the EMG values, which will act as a guide from beginning to end.