Multimodality Imaging of Sudden Cardiac Death and Acute Complications in Acute Coronary Syndrome
Abstract
1. Introduction
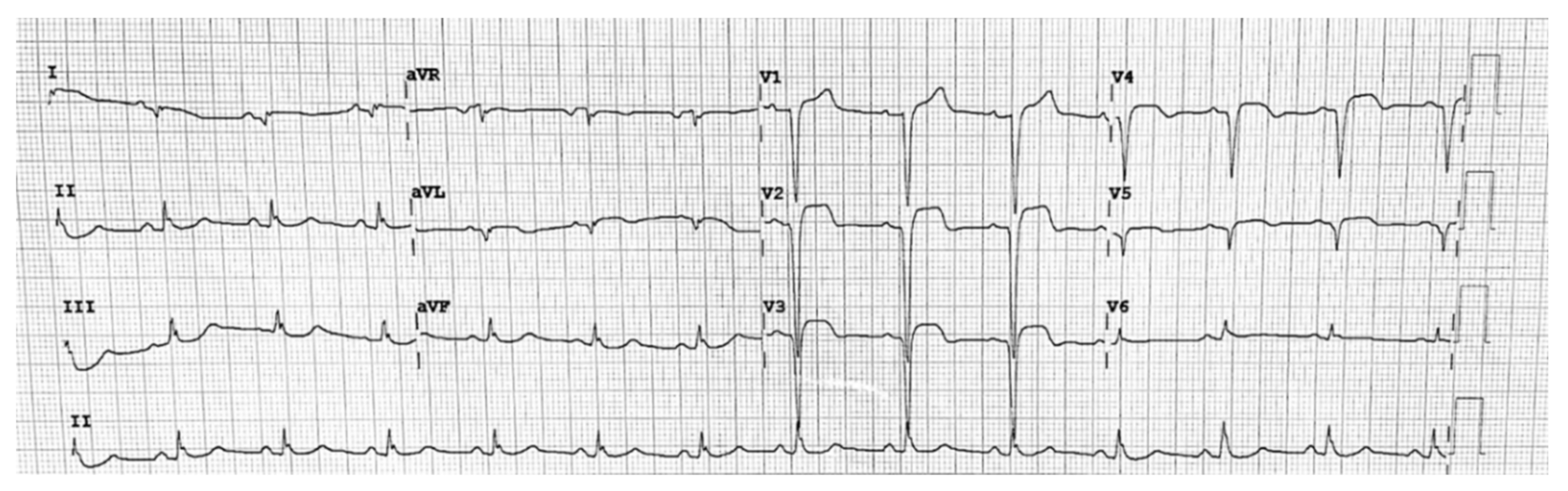
2. ECG Findings
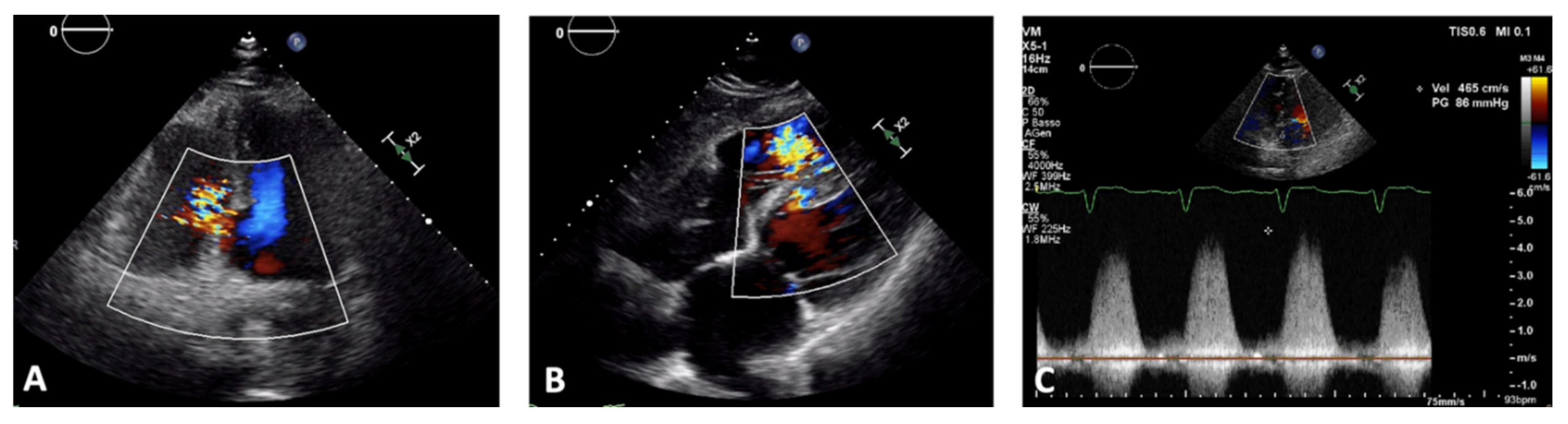
3. Echocardiography
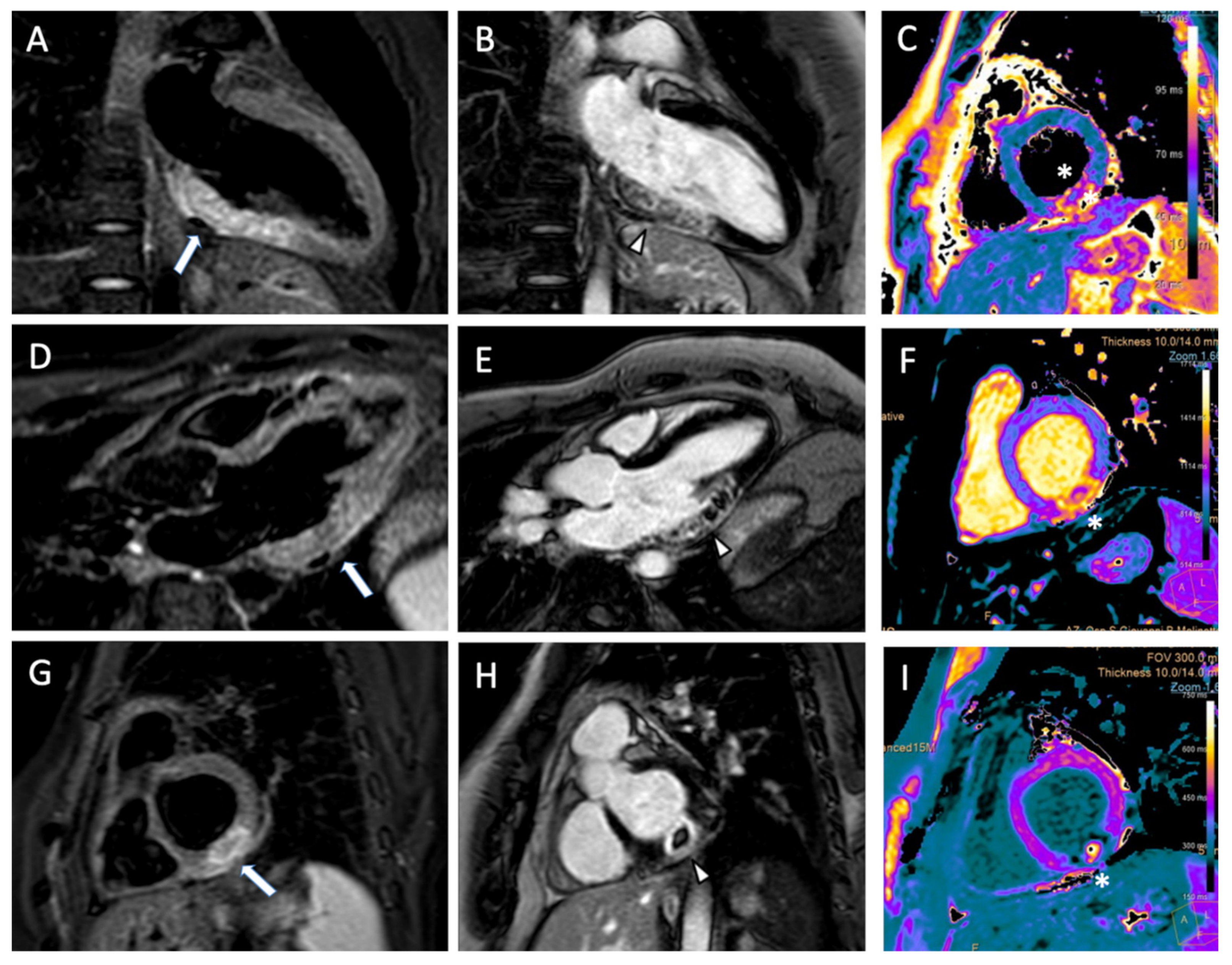
4. Cardiac Magnetic Resonance
5. Myocardial Edema and Area at Risk
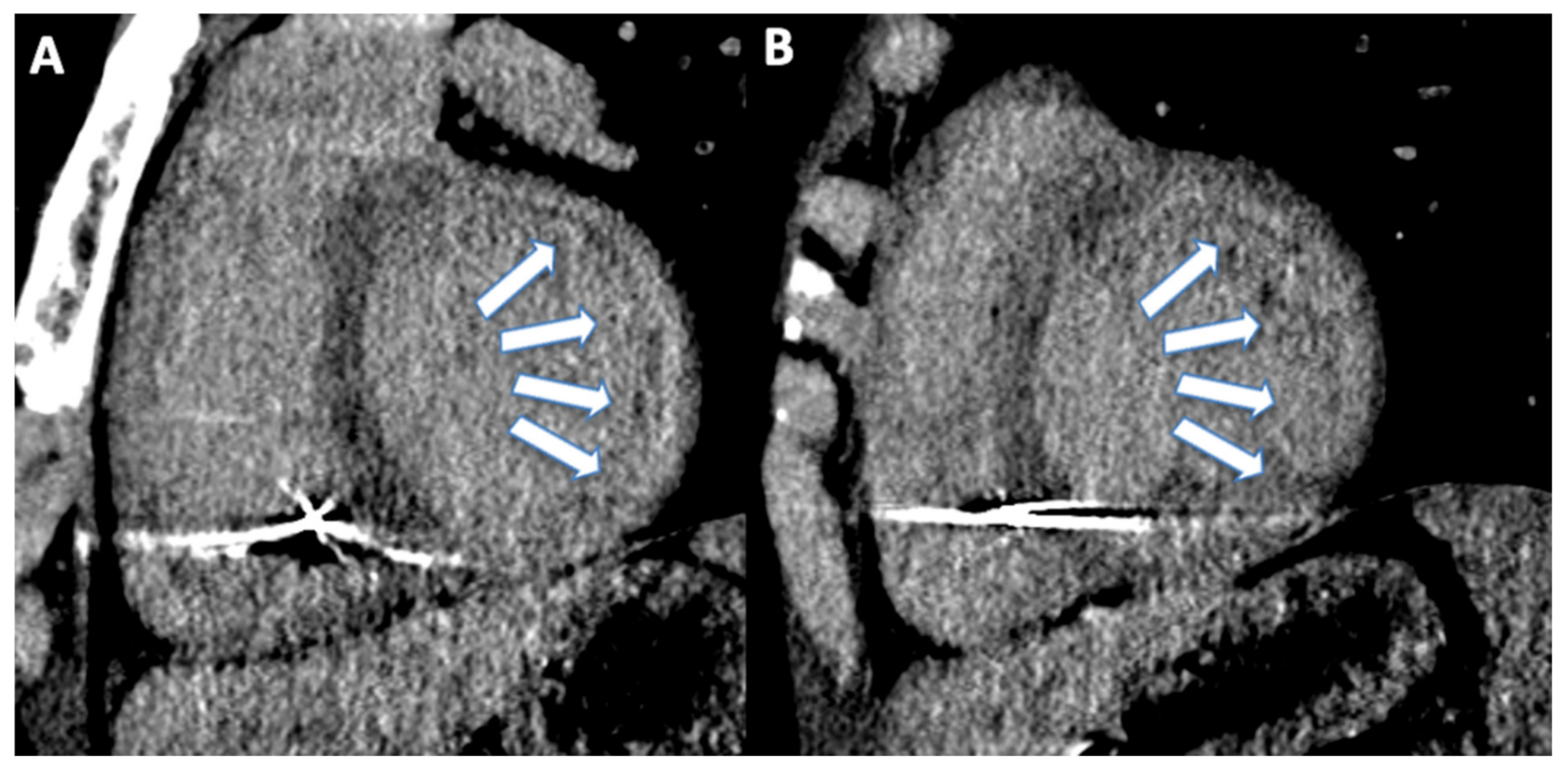
6. Microvascular Obstruction
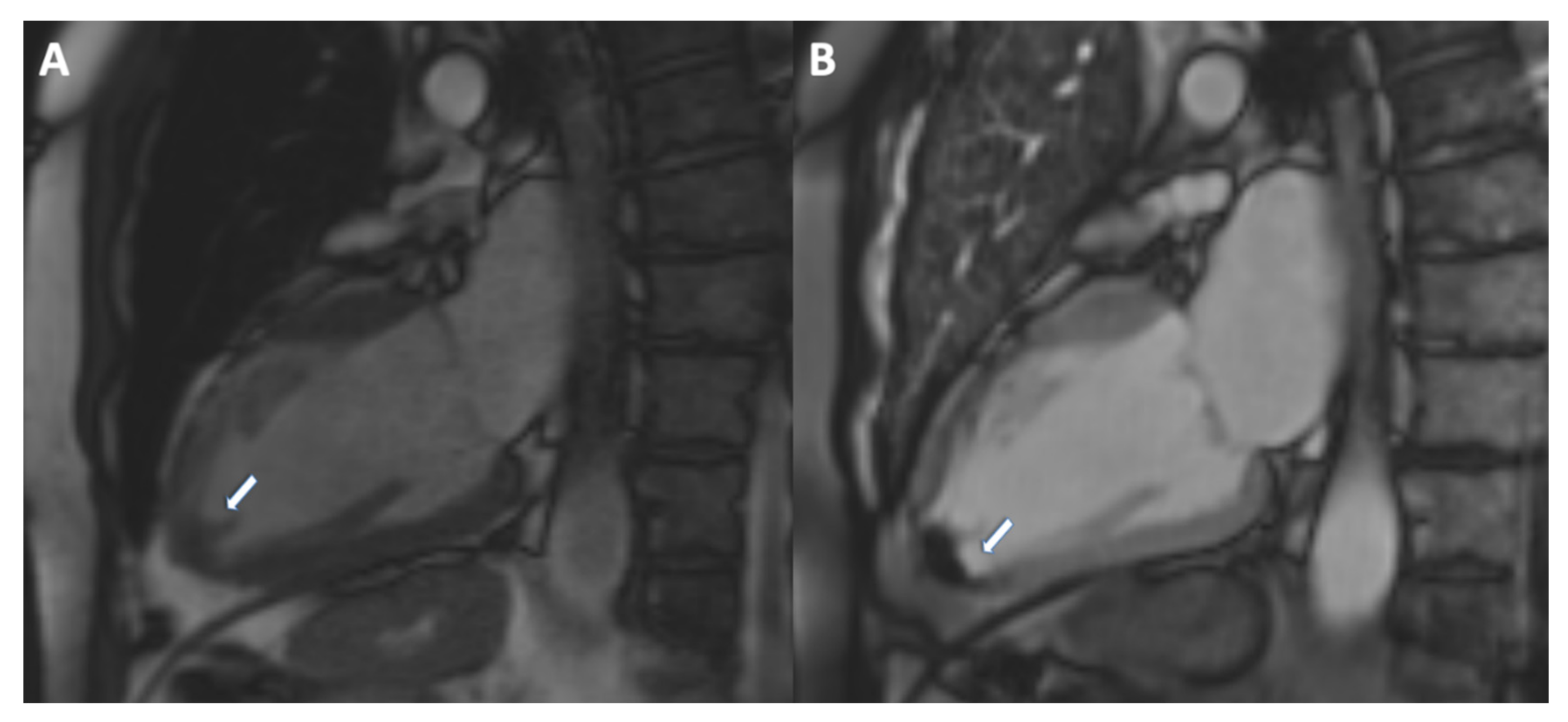
7. Ventricular Thrombus
8. Late Gadolinium Enhancement
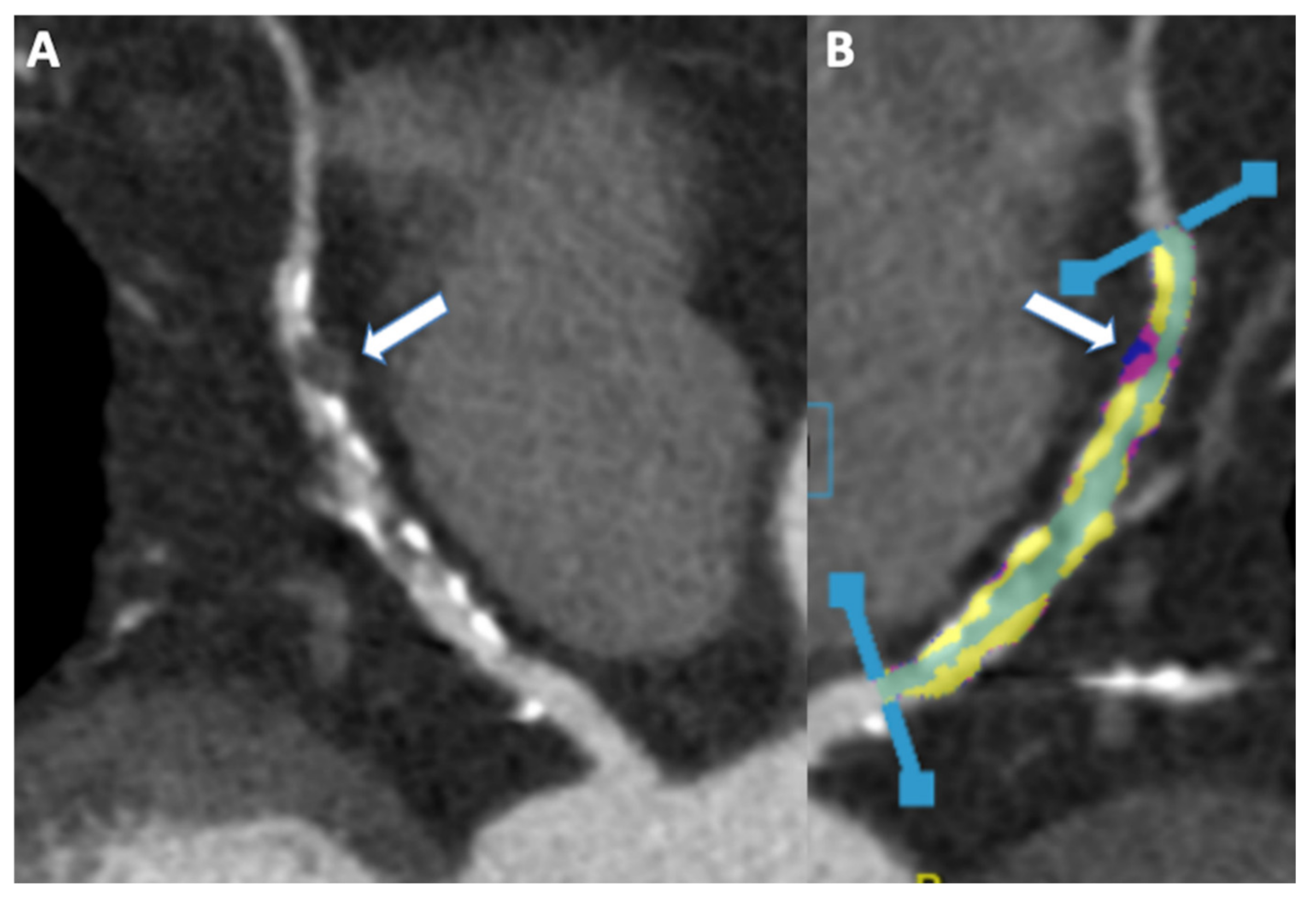
9. Cardiac Computed Tomography Angiography
10. Coronary Stenosis and Plaque
11. CCTA in Emergency
12. Application of Artificial Intelligence to Non-Invasive Multimodality Imaging
13. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vedanthan, R.; Seligman, B.; Fuster, V. Global perspective on acute coronary syndrome: A burden on the young and poor. Circ. Res. 2014, 114, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.L.; Wojdyla, D.M.; Al-Khatib, S.M.; Lokhnygina, Y.; Wallentin, L.; Armstrong, P.W.; Roe, M.T.; Ohman, E.M.; Harrington, R.A.; Alexander, J.H.; et al. Sudden Cardiac Death After Non–ST-Segment Elevation Acute Coronary Syndrome. JAMA Cardiol. 2016, 1, 73–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muscogiuri, G.; Guglielmo, M.; Serra, A.; Gatti, M.; Volpato, V.; Schoepf, U.J.; Saba, L.; Cau, R.; Faletti, R.; McGill, L.J.; et al. Multimodality Imaging in Ischemic Chronic Cardiomyopathy. J. Imaging 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Gudenkauf, B.; Hays, A.G.; Tamis-Holland, J.; Trost, J.; Ambinder, D.I.; Wu, K.C.; Arbab-Zadeh, A.; Blumenthal, R.S.; Sharma, G. Role of multimodality imaging in the assessment of myocardial infarction with nonobstructive coronary arteries: Beyond conventional coronary angiography. J. Am. Heart Assoc. 2022, 11, e022787. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Tian, J.; Lu, M. Editorial: Multimodality Imaging in Acute Coronary Syndrome. Front. Cardiovasc. Med. 2022, 9, 939428. [Google Scholar] [CrossRef]
- Pontone, G.; Guaricci, A.I.; Palmer, S.C.; Andreini, D.; Verdecchia, M.; Fusini, L.; Lorenzoni, V.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; et al. Diagnostic performance of non-invasive imaging for stable coronary artery disease: A meta-analysis. Int. J. Cardiol. 2020, 300, 276–281. [Google Scholar] [CrossRef]
- Conte, E.; Annoni, A.D.; Pontone, G.; Mushtaq, S.; Guglielmo, M.; Baggiano, A.; Volpato, V.; Agalbato, C.; Bonomi, A.; Veglia, F.; et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: A long-term follow-up study. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1170–1178. [Google Scholar] [CrossRef]
- Budoff, M.J.; Lakshmanan, S.; Toth, P.P.; Hecht, H.S.; Shaw, L.J.; Maron, D.J.; Michos, E.D.; Williams, K.A.; Nasir, K.; Choi, A.D.; et al. Cardiac CT angiography in current practice: An American society for preventive cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 9, 100318. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Tadic, M.; Moroni, F.; De Stefano, D.; Gatti, M.; Boccia, E.; Faletti, R.; Oppizzi, M.; Peretto, G.; et al. Myocardial Late Contrast Enhancement CT in Troponin-Positive Acute Chest Pain Syndrome. Radiology 2022, 302, 545–553. [Google Scholar] [CrossRef]
- van Assen, M.; Muscogiuri, G.; Caruso, D.; Lee, S.J.; Laghi, A.; De Cecco, C.N. Artificial intelligence in cardiac radiology. Radiol. Med. 2020, 125, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Chiesa, M.; Trotta, M.; Gatti, M.; Palmisano, V.; Dell’Aversana, S.; Baessato, F.; Cavaliere, A.; Cicala, G.; Loffreno, A.; et al. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis 2020, 294, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Nicol, E.D.; Weir-McCall, J.R.; Shaw, L.J.; Williamson, E. Great debates in cardiac computed tomography: OPINION: “Artifi-cial intelligence and the future of cardiovascular CT—Managing expectation and challenging hype”. J. Cardiovasc. Comput. Tomogr. 2022. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden Death Due to Cardiac Arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.A.; Fahrenbruch, C.E.; Olsufka, M.; Copass, M.K. Changing Incidence of Out-of-Hospital Ventricular Fibrillation, 1980–2000. JAMA 2002, 288, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Thomas, E.; Callaway, C.W. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008, 300, 1423–1431. [Google Scholar] [CrossRef]
- Weisfeldt, M.L.; Everson-Stewart, S.; Sitlani, C.; Rea, T.; Aufderheide, T.P.; Atkins, D.L.; Bigham, B.; Brooks, S.C.; Foerster, C.; Gray, R.; et al. Ventricular Tachyarrhythmias after Cardiac Arrest in Public versus at Home. N. Engl. J. Med. 2011, 364, 313–321. [Google Scholar] [CrossRef]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular Arrhythmia after Acute Myocardial Infarction: ‘The Perfect Storm’. Arrhythmia Electrophysiol. Rev. 2017, 6, 134–139. [Google Scholar] [CrossRef]
- Wit, A.L. Basic Electrophysiologic Mechanisms of Sudden Cardiac Death Caused by Acute Myocardial Ischemia and Infarction. Card. Electrophysiol. Clin. 2017, 9, 525–536. [Google Scholar] [CrossRef]
- Demidova, M.M.; Smith, J.G.; Höijer, C.-J.; Holmqvist, F.; Erlinge, D.; Platonov, P. Prognostic impact of early ventricular fibrillation in patients with ST-elevation myocardial infarction treated with primary PCI. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 302–311. [Google Scholar] [CrossRef]
- Khairy, P.; Thibault, B.; Talajic, M.; Dubuc, M.; Roy, D.; Guerra, P.G.; Nattel, S. Prognostic significance of ventricular arrhythmias post-myocardial infarction. Can. J. Cardiol. 2003, 19, 1393–1404. [Google Scholar] [PubMed]
- Fan, X.; Hua, W.; Xu, Y.; Ding, L.; Niu, H.; Chen, K.; Xu, B.; Zhang, S. Incidence and predictors of sudden cardiac death in patients with reduced left ventricular ejection fraction after myocardial infarction in an era of revascularisation. Heart 2014, 100, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-J.; Croft, J.B.; Giles, W.H.; Mensah, G. Sudden Cardiac Death in the United States, 1989 to 1998. Circulation 2001, 104, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Ruberman, W.; Weinblatt, E.; Goldberg, J.D.; Frank, C.W.; Chaudhary, B.S.; Shapiro, S. Ventricular premature complexes and sudden death after myocardial infarction. Circulation 1981, 64, 297–305. [Google Scholar] [CrossRef]
- Uretsky, B.F.; Sheahan, R.G. Primary prevention of sudden cardiac death in heart failure: Will the solution be shocking? J. Am. Coll. Cardiol. 1997, 30, 1589–1597. [Google Scholar] [CrossRef]
- Atkins, J.M.; Leshin, S.J.; Blomqvist, G.; Mullins, C.B. Ventricular Conduction Blocks and Sudden Death in Acute Myocardial Infarction. Potential indications for pacing. N. Engl. J. Med. 1973, 288, 281–284. [Google Scholar] [CrossRef]
- Kosmidou, I.; Redfors, B.; Dordi, R.; Dizon, J.M.; McAndrew, T.; Mehran, R.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Incidence, Predictors, and Outcomes of High-Grade Atrioventricular Block in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention (from the HORIZONS-AMI Trial). Am. J. Cardiol. 2017, 119, 1295–1301. [Google Scholar] [CrossRef]
- Kosmidou, I.; Redfors, B.; McAndrew, T. Worsening atrioventricular conduction after hospital discharge in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: The HORIZONS-AMI trial. Coron. Artery Dis. 2017, 28, 550–556. [Google Scholar] [CrossRef]
- Gang, U.J.O.; Hvelplund, A.; Pedersen, S.; Iversen, A.; Jons, C.; Abildstrøm, S.Z.; Haarbo, J.; Jensen, J.S.; Thomsen, P.E.B. High-degree atrioventricular block complicating ST-segment elevation myocardial infarction in the era of primary percutaneous coronary intervention. Europace 2012, 14, 1639–1645. [Google Scholar] [CrossRef]
- Hamm, W.; Rizas, K.D.; von Stülpnagel, L.; Vdovin, N.; Massberg, S.; Kääb, S.; Bauer, A. Implantable cardiac monitors in high-risk post-infarction patients with cardiac autonomic dysfunction and moderately reduced left ventricular ejection fraction: Design and rationale of the SMART-MI trial. Am. Heart J. 2017, 190, 34–39. [Google Scholar] [CrossRef]
- Maeda, H.; Michiue, T.; Zhu, B.-L.; Ishikawa, T.; Quan, L.; Bessho, Y.; Okazaki, S.; Kamikodai, Y.; Tsuda, K.; Komatsu, A.; et al. Potential risk factors for sudden cardiac death: An analysis of medicolegal autopsy cases. Leg. Med. 2009, 11 (Suppl. S1), S263–S265. [Google Scholar] [CrossRef] [PubMed]
- Risum, N.; Valeur, N.; Søgaard, P.; Hassager, C.; Køber, L.; Ersbøll, M. Right ventricular function assessed by 2D strain analysis predicts ventricular arrhythmias and sudden cardiac death in patients after acute myocardial infarction. Eur. Heart J.-Cardiovasc. Imaging 2017, 19, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Connolly, N.P.; Hennessey, B.; Mylotte, D. Non-ST-segment elevation myocardial infarction with evidence of transmural infarction complicated by left ventricular rupture during percutaneous coronary intervention. BMJ Case Rep. 2020, 13, e235459. [Google Scholar] [CrossRef] [PubMed]
- Olsovsky, M.R.; Topaz, O.; DiSciascio, G.; Vetrovec, G.W. Acute traumatic ventricular septal rupture. Am. Heart J. 1996, 131, 1039–1041. [Google Scholar] [CrossRef]
- Damluji, A.A.; van Diepen, S.; Katz, J.N.; Menon, V.; Tamis-Holland, J.E.; Bakitas, M.; Cohen, M.G.; Balsam, L.B.; Chikwe, J. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e16–e35. [Google Scholar] [CrossRef]
- Reddy, Y.; Al-Hijji, M.; Best, P.J.; Sinak, L.J.; Suri, R.M.; Ijioma, N.N.; Aberle, S.J.; Goyal, D.G.; Singh, M. Diagnosis of Free-Wall Rupture by Left Ventricular Angiogram After Inferior ST-Segment–Elevation Myocardial Infarction. Circulation 2015, 132, e31–e33. [Google Scholar] [CrossRef]
- Thu Kyaw, M.; Maung, Z.M. Hypokalemia-Induced Arrhythmia: A Case Series and Literature Review. Cureus 2022, 14, e22940. [Google Scholar] [CrossRef]
- El-Sherif, N.; Turitto, G. Electrolyte disorders and arrhythmogenesis. Cardiol. J. 2011, 18, 233–245. [Google Scholar]
- Diercks, D.B.; Shumaik, G.M.; Harrigan, R.A.; Brady, W.J.; Chan, T.C. Electrocardiographic manifestations: Electrolyte abnormalities. J. Emerg. Med. 2004, 27, 153–160. [Google Scholar] [CrossRef]
- Littmann, L.; Gibbs, M.A. Electrocardiographic manifestations of severe hyperkalemia. J. Electrocardiol. 2018, 51, 814–817. [Google Scholar] [CrossRef]
- Ashurst, J.; Sergent, S.R.; Sergent, B.R. Evidence-Based Management of Potassium Disorders in the Emergency Department. Emerg. Med. Pract. 2016, 18, 1–24. [Google Scholar] [PubMed]
- Olshansky, B.; Bhattacharya, S.K. Electrolytes and the ECG Intervals: Big Data and Little Insight. J. Am. Coll. Cardiol. 2019, 73, 3132–3134. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Cameli, M.; Sciaccaluga, C.; Loiacono, F.; Simova, I.; Miglioranza, M.H.; Nistor, D.O.; Bandera, F.; Emdin, M.; Giannoni, A.; Ciccone, M.M.; et al. The analysis of left atrial function predicts the severity of functional impairment in chronic heart failure: The FLASH multicenter study. Int. J. Cardiol. 2019, 286, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Porcari, A.; Pagura, L.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. A national survey on prevalence of possible echocardiographic red flags of amyloid cardiomyopathy in consecutive patients undergoing routine echocardiography: Study design and patients characterization—The first insight from the AC-TIVE Study. Eur. J. Prev. Cardiol. 2021, 29, e173–e177. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Giannoni, A.; Benfari, G.; Dini, F.L.; Pugliese, N.R.; Taddei, C.; Correale, M.; Brunetti, N.D.; Carluccio, E.; et al. Sacubitril/valsartan reduces indications for arrhythmic primary prevention in heart failure with reduced ejection fraction: Insights from DISCOVER-ARNI, a multicenter Italian register. Eur. Heart J. Open 2022, 2. [Google Scholar] [CrossRef]
- Prastaro, M.; Pirozzi, E.; Gaibazzi, N.; Paolillo, S.; Santoro, C.; Savarese, G.; Losi, M.A.; Esposito, G.; Filardi, P.P.; Trimarco, B.; et al. Expert Review on the Prognostic Role of Echocardiography after Acute Myocardial Infarction. J. Am. Soc. Echocardiogr. 2017, 30, 431–443.e2. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Chiarello, G.; Gherbesi, E.; Fusini, L.; Soldato, N.; Siena, P.; Ursi, R.; Ruggieri, R.; Guglielmo, M.; Muscogiuri, G.; et al. Coronary-specific quantification of myocardial deformation by strain echocardiography may disclose the culprit vessel in patients with non-ST-segment elevation acute coronary syndrome. Eur. Heart J. Open 2022, 2. [Google Scholar] [CrossRef]
- Takeuchi, M.; Nishikage, T.; Nakai, H.; Kokumai, M.; Otani, S.; Lang, R.M. The Assessment of Left Ventricular Twist in Anterior Wall Myocardial Infarction Using Two-dimensional Speckle Tracking Imaging. J. Am. Soc. Echocardiogr. 2007, 20, 36–44. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Davies, J.; Tuttolomondo, D.; Pontone, G.; Guaricci, A.I.; Lorenzoni, V.; Benatti, G.; Siniscalchi, C.; Pastorini, G. Association of coronary artery Doppler-echocardiography diastolic-systolic velocity ratio at rest with obstructive coronary artery stenosis on the left main or left anterior descending coronary artery. Int. J. Cardiol. 2019, 281, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Neumar, R.W.; Shuster, M.; Callaway, C.W. Part 1, Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S315–S367. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.D.; Shah, B.R.; Parsons, L.; Pollack, C.V.; French, W.J.; Canto, J.G.; Gibson, C.M.; Rogers, W.J. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008, 156, 1045–1055. [Google Scholar] [CrossRef]
- Damluji, A.A.; Forman, D.E.; van Diepen, S.; Alexander, K.P.; Page, R.L.; Hummel, S.L.; Menon, V.; Katz, J.N.; Albert, N.M.; Afilalo, J.; et al. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement from the American Heart Association. Circulation 2020, 141, e6–e32. [Google Scholar] [CrossRef]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev. Esp. Cardiol. 2017, 70, 1082. [Google Scholar] [CrossRef]
- Rogers, W.J.; Frederick, P.; Stoehr, E.; Canto, J.G.; Ornato, J.P.; Gibson, C.M.; Pollack, C.V.; Gore, J.M.; Chandra-Strobos, N.; Peterson, E.D.; et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008, 156, 1026–1034. [Google Scholar] [CrossRef]
- French, J.K.; Hellkamp, A.S.; Armstrong, P.; Cohen, E.; Kleiman, N.S.; O’Connor, C.M.; Holmes, D.R.; Hochman, J.; Granger, C.B.; Mahaffey, K.W. Mechanical Complications After Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction (from APEX-AMI). Am. J. Cardiol. 2010, 105, 59–63. [Google Scholar] [CrossRef]
- Moreyra, A.E.; Huang, M.S.; Wilson, A.C.; Deng, Y.; Cosgrove, N.M.; Kostis, J.B.; MIDAS Study Group (MIDAS 13). Trends in Incidence and Mortality Rates of Ventricular Septal Rupture During Acute Myocardial Infarction. Am. J. Cardiol. 2010, 106, 1095–1100. [Google Scholar] [CrossRef]
- Elbadawi, A.; Elgendy, I.Y.; Mahmoud, K.; Barakat, A.F.; Mentias, A.; Mohamed, A.H.; Ogunbayo, G.O.; Megaly, M.; Saad, M.; Omer, M.A.; et al. Temporal Trends and Outcomes of Mechanical Complications in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Asaumi, Y.; Yamane, T.; Nagai, T.; Miyagi, T.; Noguchi, T.; Anzai, T.; Goto, Y.; Ishihara, M.; Nishimura, K.; et al. Trends in the Clinical and Pathological Characteristics of Cardiac Rupture in Patients with Acute Myocardial Infarction Over 35 Years. J. Am. Heart Assoc. 2014, 3, e000984. [Google Scholar] [CrossRef] [PubMed]
- Sulzgruber, P.; El-Hamid, F.; Koller, L.; Forster, S.; Goliasch, G.; Wojta, J.; Niessner, A. Long-term outcome and risk prediction in patients suffering acute myocardial infarction complicated by post-infarction cardiac rupture. Int. J. Cardiol. 2017, 227, 399–403. [Google Scholar] [CrossRef]
- Matteucci, M.; Fina, D.; Jiritano, F.; Meani, P.; Blankesteijn, W.M.; Raffa, G.M.; Kowaleski, M.; Heuts, S.; Beghi, C.; Maessen, J.; et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur. Hear. J. Acute Cardiovasc. Care 2019, 8, 379–387. [Google Scholar] [CrossRef]
- Formica, F.; Mariani, S.; Singh, G.; D’Alessandro, S.; Messina, L.A.; Jones, N.; Bamodu, O.A.; Sangalli, F.; Paolini, G. Postinfarction left ventricular free wall rupture: A 17-year single-centre experience. Eur. J. Cardio-Thoracic Surg. 2018, 53, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-J.; Wang, C.-H.; Cherng, W.-J. Unruptured left ventricular pseudoaneurysm following myocardial infarction. Heart 1998, 80, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Goraya, M.H.N.; Kalsoom, S.; Almas, T.; Amin, M.K.; Hussain, N.; Awan, J.R.; Ehtesham, M.; Niaz, M.A.; Virk, H.U.H.; Filby, S.J. Simultaneous Left Ventricular Aneurysm and Ventricular Septal Rupture Complicating Delayed STEMI Presentation: A Case-Based Review of Post-MI Mechanical Complications Amid the COVID-19 Pandemic. J. Investig. Med. High Impact Case Rep. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- El Ouazzani, J.; Jandou, I. Aneurysm and pseudoaneurysm of the left ventricle. Ann. Med. Surg. 2022, 75, 103405. [Google Scholar] [CrossRef]
- Caldeira, A.; Albuquerque, D.; Coelho, M.; Côrte-Real, H. Left Ventricular Pseudoaneurysm: Imagiologic and Intraoperative Images. Circ. Cardiovasc. Imaging 2019, 12, e009500. [Google Scholar] [CrossRef]
- Alerhand, S.; Carter, J.M. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am. J. Emerg. Med. 2019, 37, 321–326. [Google Scholar] [CrossRef]
- Singh, S.; Wann, L.; Klopfenstein, H.; Hartz, A.; Brooks, H.L. Usefulness of right ventricular diastolic collapse in diagnosing cardiac tamponade and comparison to pulsus paradoxus. Am. J. Cardiol. 1986, 57, 652–656. [Google Scholar] [CrossRef]
- Gillam, L.D.; Guyer, D.E.; Gibson, T.C.; King, M.E.; Marshall, J.E.; Weyman, A.E. Hydrodynamic compression of the right atrium: A new echocardiographic sign of cardiac tamponade. Circulation 1983, 68, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Leimgruber, P.P.; Klopfenstein, H.S.; Wann, L.S.; Brooks, H.L. The hemodynamic derangement associated with right ventricular diastolic collapse in cardiac tamponade: An experimental echocardiographic study. Circulation 1983, 68, 612–620. [Google Scholar] [CrossRef]
- Jones, B.M.; Kapadia, S.R.; Smedira, N.G.; Robich, M.; Tuzcu, E.M.; Menon, V.; Krishnaswamy, A. Ventricular septal rupture complicating acute myocardial infarction: A contemporary review. Eur. Heart J. 2014, 35, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.S.; Granger, C.B.; Birnbaum, Y. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000, 101, 27–32. [Google Scholar] [CrossRef]
- Cohle, S.D.; Balraj, E.; Bell, M. Sudden death due to ventricular septal defect. Pediatr. Dev. Pathol. 1999, 2, 327–332. [Google Scholar] [CrossRef]
- Di Summa, M.; Actis Dato, G.M.; Centofanti, P.; Fortunato, G.; Patanè, F.; Di Rosa, E.; Forsennati, P.G.; La Torre, M. Ventricular septal rupture after a myocardial infarction: Clinical features and long term survival. J. Cardiovasc. Surg. 1997, 38, 589–593. [Google Scholar]
- Topaz, O.; Taylor, A.L. Interventricular septal rupture complicating acute myocardial infarction: From pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am. J. Med. 1992, 93, 683–688. [Google Scholar] [CrossRef]
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19, the pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Chamoun, A.J.; Conti, V.R.; Uretsky, B.F. Mitral regurgitation following acute myocardial infarction. Coron. Artery Dis. 2002, 13, 337–344. [Google Scholar] [CrossRef]
- Durko, A.P.; Budde, R.P.J.; Geleijnse, M.L.; Kappetein, A.P. Recognition, assessment and management of the mechanical complications of acute myocardial infarction. Heart 2018, 104, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Enriquez-Sarano, M.; Nkomo, V.T. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation 2005, 111, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N. Acute mitral regurgitation. Heart 2019, 105, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Underwood, R.D.; Sra, J.; Akhtar, M. Evaluation and treatment strategies in patients at high risk of sudden death post myocardial infarction. Clin. Cardiol. 1997, 20, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Gillespie, J.; Moss, A.J. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: An extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010, 122, 1265–1271. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Solomon, S.D.; Foster, E. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: Results from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation 2014, 130, 2278–2286. [Google Scholar]
- Stevens, S.M.; Reinier, K.; Chugh, S.S. Increased left ventricular mass as a predictor of sudden cardiac death: Is it time to put it to the test? Circ. Arrhythm. Electrophysiol. 2013, 6, 212–217. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Khan, H.; Kurl, S.; Willeit, P.; Karppi, J.; Ronkainen, K.; Di Angelantonio, E. Left Ventricular Mass and the Risk of Sudden Cardiac Death: A Population-Based Study. J. Am. Heart Assoc. 2014, 3, e001285. [Google Scholar] [CrossRef]
- Buxton, A.E.; Ellison, K.E.; Lorvidhaya, P.; Ziv, O. Left ventricular ejection fraction for sudden death risk stratification and guiding implantable cardioverter-defibrillators implantation. J. Cardiovasc. Pharmacol. 2010, 55. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, T.; Gerber, B.L.; Garot, J.; Bluemke, D.A.; Lima, J.A.; Smiseth, O.A. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: Validation against three-dimensional tagged magnetic resonance imaging. Circulation 2002, 106, 50–56. [Google Scholar] [CrossRef]
- Eek, C.; Grenne, B.; Brunvand, H.; Aakhus, S.; Endresen, K.; Smiseth, O.A.; Edvardsen, T.; Skulstad, H. Postsystolic shortening is a strong predictor of recovery of systolic function in patients with non-ST-elevation myocardial infarction. Eur. J. Echocardiogr. 2011, 12, 483–489. [Google Scholar] [CrossRef][Green Version]
- Grenne, B.; Eek, C.; Sjøli, B.; Dahlslett, T.; Hol, P.K.; Ørn, S.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Mean Strain Throughout the Heart Cycle by Longitudinal Two-Dimensional Speckle-Tracking Echocardiography Enables Early Prediction of Infarct Size. J. Am. Soc. Echocardiogr. 2011, 24, 1118–1125. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical Dispersion Assessed by Myocardial Strain in Patients After Myocardial Infarction for Risk Prediction of Ventricular Arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef]
- Dagres, N.; Hindricks, G. Risk stratification after myocardial infarction: Is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur. Heart J. 2013, 34, 1964–1971. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Gatti, M.; Dell’Aversana, S.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Mushtaq, S.; Conte, E.; Annoni, A.D.; et al. Diagnostic Accuracy of Single-shot 2-Dimensional Multisegment Late Gadolinium Enhancement in Ischemic and Nonischemic Cardiomyopathy. J. Thorac. Imaging 2020, 35, 56–63. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Gatti, M.; Dell’Aversana, S.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Andreini, D.; Mushtaq, S.; Conte, E.; Annoni, A.; et al. Image Quality and Reliability of a Novel Dark-Blood Late Gadolinium Enhancement Sequence in Ischemic Cardiomyopathy. J. Thorac. Imaging 2019, 35, 326–333. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Martini, C.; Gatti, M.; Dell’Aversana, S.; Ricci, F.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Bracciani, A.; Scafuri, S.; et al. Feasibility of late gadolinium enhancement (LGE) in ischemic cardiomyopathy using 2D-multisegment LGE combined with artificial intelligence reconstruction deep learning noise reduction algorithm. Int. J. Cardiol. 2021, 343, 164–170. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Rehwald, W.G.; Schoepf, U.J.; Suranyi, P.; Litwin, S.E.; De Cecco, C.N.; Wichmann, J.L.; Mangold, S.; Caruso, D.; Fuller, S.R.; et al. T(Rho) and magnetization transfer and INvErsion recovery (TRAMINER)-prepared imaging: A novel contrast-enhanced flow-independent dark-blood technique for the evaluation of myocardial late gadolinium enhancement in patients with myocardial infarction. J. Magn. Reson. Imaging 2017, 45, 1429–1437. [Google Scholar] [CrossRef]
- Roes, S.D.; Borleffs, C.J.W.; van der Geest, R.J.; Westenberg, J.J.; Marsan, N.A.; Kaandorp, T.A.; Reiber, J.H.; Zeppenfeld, K.; Lamb, H.J.; de Roos, A.; et al. Infarct Tissue Heterogeneity Assessed with Contrast-Enhanced MRI Predicts Spontaneous Ventricular Arrhythmia in Patients with Ischemic Cardiomyopathy and Implantable Cardioverter-Defibrillator. Circ. Cardiovasc. Imaging 2009, 2, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Bertella, E.; Loguercio, M.; Guglielmo, M.; Baggiano, A.; Aquaro, G.D.; Mushtaq, S.; Salerni, S.; Gripari, P.; et al. Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A mid-term follow-up study. Eur. Radiol. 2016, 26, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Ricci, F.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; De Stasio, V.; Di Donna, C.; Spiritigliozzi, L.; Chiocchi, M.; Lee, S.J.; et al. Cardiac Magnetic Resonance Tissue Characterization in Ischemic Cardiomyopathy. J. Thorac. Imaging 2022, 37, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Baggiano, A.; Bertella, E.; Mushtaq, S.; Conte, E.; Beltrama, V.; Guaricci, A.I.; Pepi, M. Functional Relevance of Coronary Artery Disease by Cardiac Magnetic Resonance and Cardiac Computed Tomography: Myocardial Perfusion and Fractional Flow Reserve. BioMed Res. Int. 2015, 2015, 297696. [Google Scholar] [CrossRef]
- Kim, H.W.; Van Assche, L.; Jennings, R.B.; Wince, W.B.; Jensen, C.J.; Rehwald, W.G.; Wendell, D.C.; Bhatti, L.; Spatz, D.M.; Parker, M.A.; et al. Relationship of T2-Weighted MRI Myocardial Hyperintensity and the Ischemic Area-At-Risk. Circ. Res. 2015, 117, 254–265. [Google Scholar] [CrossRef]
- Srichai, M.B.; Lim, R.P.; Lath, N.; Babb, J.; Axel, L.; Kim, D. Diagnostic Performance of Dark-Blood T2-Weighted CMR for Evaluation of Acute Myocardial Injury. Investig. Radiol. 2013, 48, 24–31. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Muscogiuri, G.; Varga-Szemes, A.; Schoepf, U.J. Cutting edge clinical applications in cardiovascular magnetic resonance. World J. Radiol. 2017, 9, 1–4. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Suranyi, P.; Schoepf, U.J. Cardiac Magnetic Resonance T1-Mapping of the Myocardium: Technical Background and Clinical Relevance. J. Thorac. Imaging 2018, 33, 71–80. [Google Scholar] [CrossRef]
- Giri, S.; Chung, Y.-C.; Merchant, A.; Mihai, G.; Rajagopalan, S.; Raman, S.V.; Simonetti, O.P. T2 quantification for improved detection of myocardial edema. J. Cardiovasc. Magn. Reson. 2009, 11, 56. [Google Scholar] [CrossRef]
- Bulluck, H.; White, S.K.; Rosmini, S.; Bhuva, A.N.; Treibel, T.A.; Fontana, M.; Abdel-Gadir, A.; Herrey, A.S.; Manisty, C.H.; Wan, S.M.Y.; et al. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. J. Cardiovasc. Magn. Reson. 2015, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Aletras, A.H.; Arai, A.E. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Col.l Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; García-Ruiz, J.M.; García-Álvarez, A.; Fernández-Jiménez, R.; Sánchez-González, J.; Rossello, X.; Gómez-Talavera, S.; López-Martín, G.J.; Pizarro, G.; Fuster, V.; et al. Accuracy of Area at Risk Quantification by Cardiac Magnetic Resonance According to the Myocardial Infarction Territory. Rev. Española Cardiol. 2017, 70, 323–330. [Google Scholar] [CrossRef]
- Zorzi, A.; Mattesi, G.; Baldi, E.; Toniolo, M.; Guerra, F.; Cauti, F.M.; Cipriani, A.; De Lazzari, M.; Muser, D.; Stronati, G.; et al. Prognostic Role of Myocardial Edema as Evidenced by Early Cardiac Magnetic Resonance in Survivors of Out-of-Hospital Cardiac Arrest: A Multicenter Study. J. Am. Heart Assoc. 2021, 10, e021861. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Susana, A.; De Lazzari, M.; Migliore, F.; Vescovo, G.; Scarpa, D.; Baritussio, A.; Tarantini, G.; Cacciavillani, L.; Giorgi, B.; et al. Diagnostic value and prognostic implications of early cardiac magnetic resonance in survivors of out-of-hospital cardiac arrest. Heart Rhythm 2018, 15, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Guaricci, A.I. Association Between Haptoglobin Phenotype and Microvascular Obstruction in Patients With STEMI: A Cardiac Magnetic Resonance Study. JACC Cardiovasc. Imaging 2019, 12, 1007–1017. [Google Scholar] [CrossRef]
- Abbas, A.; Matthews, G.H.; Brown, I.W.; Shambrook, J.; Peebles, C.R.; Harden, S.P. Cardiac MR assessment of microvascular obstruction. Br. J. Radiol. 2015, 88, 20140470. [Google Scholar] [CrossRef]
- Chen, B.H.; An, D.A.; He, J.; Xu, J.R.; Wu, L.M.; Pu, J. Myocardial Extracellular Volume Fraction Allows Differentiation of Reversible Versus Irreversible Myocardial Damage and Prediction of Adverse Left Ventricular Remodeling of ST-Elevation Myocardial Infarction. J. Magn. Reson. Imaging 2020, 52, 476–487. [Google Scholar] [CrossRef]
- Nijveldt, R.; Hofman, M.B.M.; Hirsch, A.; Beek, A.M.; Umans, V.A.W.M.; Algra, P.R.; Piek, J.J.; Van Rossum, A.C. Assessment of Microvascular Obstruction and Prediction of Short-term Remodeling after Acute Myocardial Infarction: Cardiac MR Imaging Study. Radiology 2009, 250, 363–370. [Google Scholar] [CrossRef]
- Ito, H.; Tomooka, T.; Sakai, N.; Yu, H.; Higashino, Y.; Fujii, K.; Masuyama, T.; Kitabatake, A.; Minamino, T. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 1992, 85, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Maruyama, A.; Iwakura, K. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 1996, 93, 223–228. [Google Scholar] [CrossRef] [PubMed]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Hamirani, Y.S.; Wong, A.; Kramer, C.M.; Salerno, M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: A systematic review and meta-analysis. JACC Cardiovasc. Imaging 2014, 7, 940–952. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, D.P.; Ariff, B.; Neuwirth, C.; Tan, Y.; Durighel, G.; Cook, S.A. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart 2010, 96, 1885–1891. [Google Scholar] [CrossRef]
- Mather, A.N.; Fairbairn, T.A.; Ball, S.G.; Greenwood, J.P.; Plein, S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart 2011, 97, 453–459. [Google Scholar] [CrossRef]
- Cokic, I.; Kali, A.; Wang, X.; Yang, H.-J.; Tang, R.L.Q.; Thajudeen, A.; Shehata, M.; Amorn, A.M.; Liu, E.; Stewart, B.; et al. Iron Deposition following Chronic Myocardial Infarction as a Substrate for Cardiac Electrical Anomalies: Initial Findings in a Canine Model. PLoS ONE 2013, 8, e73193. [Google Scholar] [CrossRef][Green Version]
- Delewi, R.; Zijlstra, F.; Piek, J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012, 98, 1743–1749. [Google Scholar] [CrossRef]
- Motwani, M.; Kidambi, A.; Herzog, B.A.; Uddin, A.; Greenwood, J.P.; Plein, S. MR Imaging of Cardiac Tumors and Masses: A Review of Methods and Clinical Applications. Radiology 2013, 268, 26–43. [Google Scholar] [CrossRef]
- Roifman, I.; Connelly, K.A.; Wright, G.A.; Wijeysundera, H.C. Echocardiography vs Cardiac Magnetic Resonance Imaging for the Diagnosis of Left Ventricular Thrombus: A Systematic Review. Can. J. Cardiol. 2015, 31, 785–791. [Google Scholar] [CrossRef]
- Caspar, T.; El Ghannudi, S.; Ohana, M.; Labani, A.; Lawson, A.; Ohlmann, P.; Morel, O.; De Mathelin, M.; Roy, C.; Gangi, A.; et al. Magnetic resonance evaluation of cardiac thrombi and masses by T1 and T2 mapping: An observational study. Int. J. Cardiovasc. Imaging 2017, 33, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Chan, M.H.H.; Paradies, V.; Yellon, R.L.; Ho, H.H.; Chan, M.Y.; Chin, C.W.L.; Tan, J.W.; Hausenloy, D.J. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: A meta-analysis. J. Cardiovasc. Magn. Reson. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, R.S.; Visser, C.A.; Fuster, V. Intracardiac Thrombi and Systemic Embolization. Ann. Intern. Med. 1986, 104, 689. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Masci, P.G.; Muscogiuri, G. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: An international Registry. Europace 2021, 23, 1072–1083. [Google Scholar] [CrossRef]
- Al’Aref, S.J.; Altibi, A.M.; Malkawi, A.; Mansour, M.; Baskaran, L.; Masri, A.; Rahmouni, H.; Abete, R.; Andreini, D.; et al.; Writing Committee Cardiac magnetic resonance for prophylactic implantable-cardioverter defibrillator therapy international study: Prognostic value of cardiac magnetic resonance-derived right ventricular parameters substudy. Eur. Heart J.-Cardiovasc. Imaging 2022. [Google Scholar] [CrossRef]
- Merlo, M.; Gagno, G.; Baritussio, A. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail Rev. 2022. [Google Scholar] [CrossRef]
- Bogun, F.; Desjardins, B.; Crawford, T.; Good, E.; Jongnarangsin, K.; Oral, H.; Chugh, A.; Pelosi, F.; Morady, F. Post-Infarction Ventricular Arrhythmias Originating in Papillary Muscles. J. Am. Coll. Cardiol. 2008, 51, 1794–1802. [Google Scholar] [CrossRef]
- Robbers, L.F.H.J.; Delewi, R.; Nijveldt, R.; Hirsch, A.; Beek, A.M.; Kemme, M.; Van Beurden, Y.; Van Der Laan, A.M.; Van Der Vleuten, P.A.; Tio, R.A.; et al. Myocardial infarct heterogeneity assessment by late gadolinium enhancement cardiovascular magnetic resonance imaging shows predictive value for ventricular arrhythmia development after acute myocardial infarction. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 1150–1158. [Google Scholar] [CrossRef]
- Kim, R.J.; Chen, E.-L.; Lima, J.A.; Judd, R.M. Myocardial Gd-DTPA Kinetics Determine MRI Contrast Enhancement and Reflect the Extent and Severity of Myocardial Injury After Acute Reperfused Infarction. Circulation 1996, 94, 3318–3326. [Google Scholar] [CrossRef]
- Kim, R.J.; Fieno, D.S.; Parrish, T.; Harris, K.; Chen, E.-L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI Delayed Contrast Enhancement to Irreversible Injury, Infarct Age, and Contractile Function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef]
- Kim, R.J.; Shah, D.J.; Judd, R.M. How We Perform Delayed Enhancement Imaging. J. Cardiovasc. Magn. Reson. 2003, 5, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Holtackers, R.J.; Van De Heyning, C.M.; Nazir, M.S.; Rashid, I.; Ntalas, I.; Rahman, H.; Botnar, R.M.; Chiribiri, A. Clinical value of dark-blood late gadolinium enhancement cardiovascular magnetic resonance without additional magnetization preparation. J. Cardiovasc. Magn. Reson. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Good, E.; Desjardins, B.; Jongnarangsin, K.; Oral, H.; Chugh, A.; Ebinger, M.; Pelosi, F.; Morady, F.; Bogun, F. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: A comparison with fascicular arrhythmias. Heart Rhythm 2008, 5, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2020, 31, 1100–1109. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee, M.; Gulati, M.; Levy, P.D. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Cardiovasc. Comput. Tomogr. 2022, 16, 54–122. [Google Scholar]
- Guaricci, A.I.; Maffei, E.; Brunetti, N.D.; Montrone, D.; Di Biase, L.; Tedeschi, C.; Gentile, G.; Macarini, L.; Midiri, M.; Cademartiri, F.; et al. Heart rate control with oral ivabradine in computed tomography coronary angiography: A randomized comparison of 7.5 mg vs. 5 mg regimen. Int. J. Cardiol. 2013, 168, 362–368. [Google Scholar] [CrossRef]
- Maffei, E.; Seitun, S.; Martini, C.; Palumbo, A.; Tarantini, G.; Berti, E.; Grilli, R.; Tedeschi, C.; Messalli, G.; Guaricci, A.; et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart 2010, 96, 1973–1979. [Google Scholar] [CrossRef]
- Maffei, E.; Seitun, S.; Martini, C.; Aldrovandi, A.; Cervellin, G.; Tedeschi, C.; Guaricci, A.I.; Messalli, G.; Catalano, O.; Cademartiri, F. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. La Radiol. Med. 2011, 116, 690–705. [Google Scholar] [CrossRef]
- Esposito, A.; Francone, M.; Andreini, D. SIRM-SIC appropriateness criteria for the use of Cardiac Computed Tomography. Part 1, Congenital heart diseases, primary prevention, risk assessment before surgery, suspected CAD in symptomatic patients, plaque and epicardial adipose tissue characterization, and functional assessment of stenosis. Radiol. Med. 2021, 126, 1236–1248. [Google Scholar]
- Carrabba, N.; Pontone, G.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. Appropriateness criteria for the use of cardiac computed tomography, SIC-SIRM part 2, acute chest pain evaluation; stent and coronary artery bypass graft patency evaluation; planning of coronary revascularization and transcatheter valve procedures; cardiomyopathies, electrophysiological applications, cardiac masses, cardio-oncology and pericardial diseases evaluation. J. Cardiovasc. Med. 2022, 23, 290–303. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Arcadi, T.; Brunetti, N.D.; Maffei, E.; Montrone, D.; Martini, C.; De Luca, M.; De Rosa, F.; Cocco, D.; Midiri, M.; et al. Carotid intima media thickness and coronary atherosclerosis linkage in symptomatic intermediate risk patients evaluated by coronary computed tomography angiography. Int. J. Cardiol. 2014, 176, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Pontone, G.; Fusini, L.; De Luca, M.; Cafarelli, F.P.; Guglielmo, M.; Baggiano, A.; Beltrama, V.; Muscogiuri, G.; Mushtaq, S.; et al. Additional value of inflammatory biomarkers and carotid artery disease in prediction of significant coronary artery disease as assessed by coronary computed tomography angiography. Eur. Heart J.-Cardiovasc. Imaging 2016, 18, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Bastarrika, G.; Arraiza, M.; Enrici, M.M.; Pueyo, J.; Muscogiuri, G.; Fina, P.; Anselmi, A.; Di Girolamo, M.; David, V. Dual source CT: State of the art in the depiction of coronary arteries anatomy, anatomical variants and myocardial segments. Minerva Cardioangiol. 2012, 60, 133–146. [Google Scholar] [PubMed]
- Esposito, A.; Palmisano, A.; Barbera, M. Cardiac Computed Tomography in Troponin-Positive Chest Pain: Sometimes the Answer Lies in the Late Iodine Enhancement or Extracellular Volume Fraction Map. JACC Cardiovasc. Imaging 2019, 12, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O. What atherosclerosis findings can CT see in sudden coronary death: Plaque rupture versus plaque erosion. J. Cardiovasc Comput. Tomogr. 2020, 14, 214–218. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Min, J.K.; Shaw, L.J.; Devereux, R.B.; Okin, P.M.; Weinsaft, J.W.; Russo, D.J.; Lippolis, N.J.; Berman, D.S.; Callister, T.Q. Prognostic Value of Multidetector Coronary Computed Tomographic Angiography for Prediction of All-Cause Mortality. J. Am. Coll. Cardiol. 2007, 50, 1161–1170. [Google Scholar] [CrossRef]
- Cury, R.C.; Blankstein, R.; Leipsic, J. CAD-RADS 2.0—2022 Coronary Artery Disease—Reporting and Data System an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America society of cardiovascular imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2022, in press. [Google Scholar]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Fazzari, F.; Berzovini, C.; et al. Quantitative vs. qualitative evaluation of static stress computed tomography perfusion to detect haemodynamically significant coronary artery disease. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Baggiano, A.; Fusini, L.; Del Torto, A. Sequential Strategy Including FFR(CT) Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Liu, T.; Mayrhofer, T. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef]
- Hoffmann, U.; Bamberg, F.; Chae, C.U.; Nichols, J.H.; Rogers, I.S.; Seneviratne, S.K.; Truong, Q.A.; Cury, R.C.; Abbara, S.; Shapiro, M.D.; et al. Coronary Computed Tomography Angiography for Early Triage of Patients with Acute Chest Pain: The ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) Trial. J. Am. Coll. Cardiol. 2009, 53, 1642–1650. [Google Scholar] [CrossRef]
- Gautam, N.; Saluja, P.; Malkawi, A.; Rabbat, M.G.; Al-Mallah, M.H.; Pontone, G.; Zhang, Y.; Lee, B.C.; Al’Aref, S.J. Current and Future Applications of Artificial Intelligence in Coronary Artery Disease. Healthcare 2022, 10, 232. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chiesa, M.; Baggiano, A.; Spadafora, P.; De Santis, R.; Guglielmo, M.; Scafuri, S.; Fusini, L.; Mushtaq, S.; Conte, E.; et al. Diagnostic performance of deep learning algorithm for analysis of computed tomography myocardial perfusion. Eur. J. Pediatr. 2022, 49, 3119–3128. [Google Scholar] [CrossRef]
- Tat, E.; Bhatt, D.L.; Rabbat, M.G. Addressing bias: Artificial intelligence in cardiovascular medicine. Lancet Digit. Health 2020, 2, e635–e636. [Google Scholar] [CrossRef]
- Narang, A.; Mor-Avi, V.; Prado, A.; Volpato, V.; Prater, D.; Tamborini, G.; Fusini, L.; Pepi, M.; Goyal, N.; Addetia, K.; et al. Machine learning based automated dynamic quantification of left heart chamber volumes. Eur. Heart J.-Cardiovasc. Imaging 2018, 20, 541–549. [Google Scholar] [CrossRef]
- Kusunose, K.; Haga, A.; Abe, T.; Sata, M. Utilization of Artificial Intelligence in Echocardiography. Circ. J. 2019, 83, 1623–1629. [Google Scholar] [CrossRef]
- Penso, M.; Pepi, M.; Mantegazza, V.; Cefalù, C.; Muratori, M.; Fusini, L.; Gripari, P.; Ali, S.G.; Caiani, E.G.; Tamborini, G. Machine Learning Prediction Models for Mitral Valve Repairability and Mitral Regurgitation Recurrence in Patients Undergoing Surgical Mitral Valve Repair. Bioengineering 2021, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Moccia, S.; Banali, R.; Martini, C.; Muscogiuri, G.; Pontone, G.; Pepi, M.; Caiani, E.G. Development and testing of a deep learning-based strategy for scar segmentation on CMR-LGE images. Magn. Reson. Mater. Physics, Biol. Med. 2018, 32, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. BioMed Res. Int. 2020, 2020, 6649410. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.D.; Marques, H.; Kumar, V. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J Cardiovasc Comput Tomogr 2021, 15, 470–476. [Google Scholar] [CrossRef]
- Griffin, W.F.; Choi, A.D.; Riess, J.S. AI Evaluation of Stenosis on Coronary CT Angiography, Comparison with Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2022, in press. [Google Scholar] [CrossRef]
- Motwani, M.; Dey, D.; Berman, D.S.; Germano, G.; Achenbach, S.; Al-Mallah, M.; Andreini, D.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: A 5-year multicentre prospective registry analysis. Eur. Heart J. 2016, 38, 500–507. [Google Scholar] [CrossRef]
- Van Rosendael, A.R.; Maliakal, G.; Kolli, K.K.; Beecy, A.; Al’Aref, S.J.; Dwivedi, A.; Singh, G.; Panday, M.; Kumar, A.; Ma, X. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J. Cardiovasc. Comput. Tomogr. 2018, 12, 204–209. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscogiuri, G.; Guaricci, A.I.; Soldato, N.; Cau, R.; Saba, L.; Siena, P.; Tarsitano, M.G.; Giannetta, E.; Sala, D.; Sganzerla, P.; et al. Multimodality Imaging of Sudden Cardiac Death and Acute Complications in Acute Coronary Syndrome. J. Clin. Med. 2022, 11, 5663. https://doi.org/10.3390/jcm11195663
Muscogiuri G, Guaricci AI, Soldato N, Cau R, Saba L, Siena P, Tarsitano MG, Giannetta E, Sala D, Sganzerla P, et al. Multimodality Imaging of Sudden Cardiac Death and Acute Complications in Acute Coronary Syndrome. Journal of Clinical Medicine. 2022; 11(19):5663. https://doi.org/10.3390/jcm11195663
Chicago/Turabian StyleMuscogiuri, Giuseppe, Andrea Igoren Guaricci, Nicola Soldato, Riccardo Cau, Luca Saba, Paola Siena, Maria Grazia Tarsitano, Elisa Giannetta, Davide Sala, Paolo Sganzerla, and et al. 2022. "Multimodality Imaging of Sudden Cardiac Death and Acute Complications in Acute Coronary Syndrome" Journal of Clinical Medicine 11, no. 19: 5663. https://doi.org/10.3390/jcm11195663
APA StyleMuscogiuri, G., Guaricci, A. I., Soldato, N., Cau, R., Saba, L., Siena, P., Tarsitano, M. G., Giannetta, E., Sala, D., Sganzerla, P., Gatti, M., Faletti, R., Senatieri, A., Chierchia, G., Pontone, G., Marra, P., Rabbat, M. G., & Sironi, S. (2022). Multimodality Imaging of Sudden Cardiac Death and Acute Complications in Acute Coronary Syndrome. Journal of Clinical Medicine, 11(19), 5663. https://doi.org/10.3390/jcm11195663









