Statin Therapy and the Risk of Viral Infection: A Retrospective Population-Based Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Design and Patients
2.3. Study Outcomes
2.4. Statistical Analyses
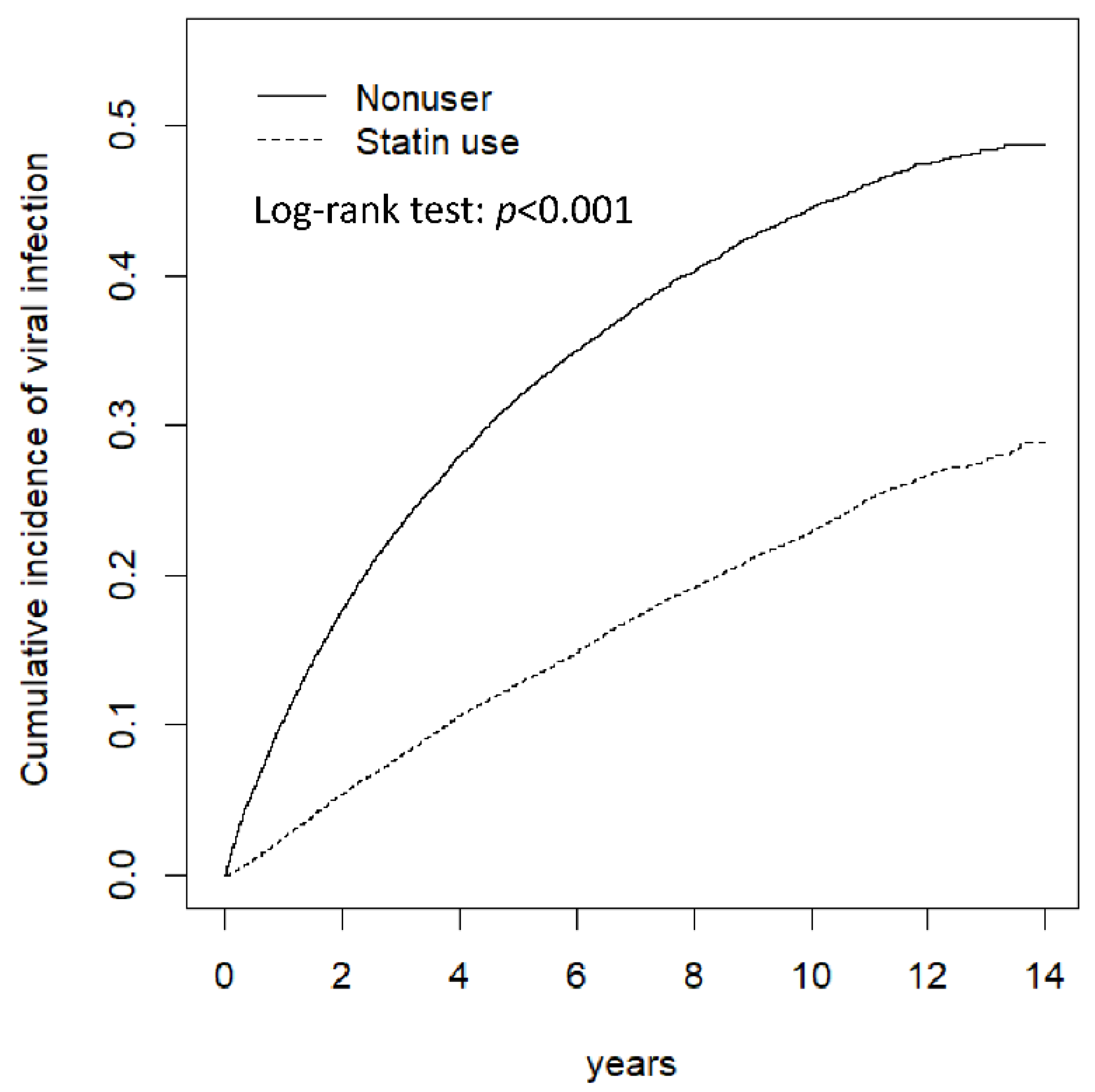
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; De Mets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins—Basic research and clinical perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef]
- Dehnavi, S.; Sohrabi, N.; Sadeghi, M.; Lansberg, P.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Statins and autoimmunity: State-of-the-art. Pharmacol. Ther. 2020, 214, 107614. [Google Scholar] [CrossRef]
- Mach, F. Statins as immunomodulatory agents. Circulation 2004, 109, II15–II17. [Google Scholar] [CrossRef]
- Shahoei, S.H.; Nelson, E.R. Nuclear receptors, cholesterol homeostasis and the immune system. J. Steroid. Biochem. Mol. Biol. 2019, 191, 105364. [Google Scholar] [CrossRef]
- Fessler, M.B. The Intracellular Cholesterol Landscape: Dynamic Integrator of the Immune Response. Trends Immunol. 2016, 37, 819–830. [Google Scholar] [CrossRef]
- Van den Broeke, C.; Jacob, T.; Favoreel, H.W. Rho’ing in and out of cells: Viral interactions with Rho GTPase signaling. Small GTPases 2014, 5, e28318. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Bianconi, V.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Antiviral effects of statins. Prog. Lipid Res. 2020, 79, 101054. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, M.L.; Thomas, A.R.; Kamimoto, L.; Reingold, A.; Gershman, K.; Meek, J.; Farley, M.M.; Ryan, P.; Lynfield, R.; Baumbach, J.; et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: A multistate study. J. Infect. Dis. 2012, 205, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Atamna, A.; Babitch, T.; Bracha, M.; Sorek, N.; Haim, B.Z.; Elis, A.; Bishara, J.; Avni, T. Statins and outcomes of hospitalized patients with laboratory-confirmed 2017–2018 influenza. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin Epidemiol 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gomez, J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Mehrbod, P.; Hair-Bejo, M.; Tengku Ibrahim, T.A.; Omar, A.R.; El Zowalaty, M.; Ajdari, Z.; Ideris, A. Simvastatin modulates cellular components in influenza A virus-infected cells. Int. J. Mol. Med. 2014, 34, 61–73. [Google Scholar] [CrossRef]
- Shrivastava-Ranjan, P.; Flint, M.; Bergeron, E.; McElroy, A.K.; Chatterjee, P.; Albarino, C.G.; Nichol, S.T.; Spiropoulou, C.F. Statins Suppress Ebola Virus Infectivity by Interfering with Glycoprotein Processing. mBio 2018, 9, e00660-18. [Google Scholar] [CrossRef]
- Rodriguez-Nava, G.; Trelles-Garcia, D.P.; Yanez-Bello, M.A.; Chung, C.W.; Trelles-Garcia, V.P.; Friedman, H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: A retrospective cohort study. Crit. Care 2020, 24, 429. [Google Scholar] [CrossRef]
- Kwong, J.C.; Li, P.; Redelmeier, D.A. Influenza morbidity and mortality in elderly patients receiving statins: A cohort study. PLoS ONE 2009, 4, e8087. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cruz, J.; Costanzo, A.; Terajima, M.; Ennis, F.A. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol. 2010, 264, 71–77. [Google Scholar] [CrossRef]
- Rothwell, C.; Lebreton, A.; Young Ng, C.; Lim, J.Y.; Liu, W.; Vasudevan, S.; Labow, M.; Gu, F.; Gaither, L.A. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 2009, 389, 8–19. [Google Scholar] [CrossRef]
- Schonbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents? Circulation 2004, 109, II18–II26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Wang, Z.; Ersing, I.; Nobre, L.; Guo, R.; Jiang, S.; Trudeau, S.; Zhao, B.; Weekes, M.P.; Gewurz, B.E. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. PLoS Pathog. 2019, 15, e1008030. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Uota, S.; Saitoh, Y.; Takahashi, M.; Sugimoto, H.; Amet, T.; Arai, A.; Miura, O.; Yamamoto, N.; Yamaoka, S. Role for protein geranylgeranylation in adult T-cell leukemia cell survival. Exp. Cell Res. 2009, 315, 141–150. [Google Scholar] [CrossRef]
- Liu, A.; Ildefonso, C.J.; Bond, W.S.; Hurwitz, M.Y.; Hurwitz, R.L. Inhibitors of metalloprotease, gamma-sectretase, protein kinase C and Rho kinase inhibit wild-type adenoviral replication. PLoS ONE 2020, 15, e0236175. [Google Scholar] [CrossRef]
- Young, J.M.; Zine El Abidine, A.; Gomez-Martinez, R.A.; Ozbun, M.A. The Known and Potential Intersections of Rab-GTPases in Human Papillomavirus Infections. Front. Cell Dev. Biol. 2019, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Su, W.J.; Yen, Y.F.; Pan, S.W.; Chuang, P.H.; Feng, J.Y.; Chou, K.T.; Yang, K.Y.; Lee, Y.C.; Chen, T.J. Statin Use Is Associated With a Lower Risk of TB. Chest 2017, 152, 598–606. [Google Scholar] [CrossRef]
- Kang, E.Y.; Chen, T.H.; Garg, S.J.; Sun, C.C.; Kang, J.H.; Wu, W.C.; Hung, M.J.; Lai, C.C.; Cherng, W.J.; Hwang, Y.S. Association of Statin Therapy With Prevention of Vision-Threatening Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 363–371. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Yang, H.Y.; Wu, C.Y.; Tsai, C.Y.; Chen, C.Y.; Hsiao, C.C.; Hsu, H.H.; Tian, Y.C.; Yen, C.L. Does Statin Therapy Reduce the Risks of Mortality and Major Adverse Cardiac and Cerebrovascular Events in Young Adults with End-Stage Renal Disease? Population-Based Cohort Study. J. Clin. Med. 2021, 10, 2097. [Google Scholar] [CrossRef]
| Propensity Score Matched | |||
|---|---|---|---|
| Variable | Statin | Standardized Mean Differences § | |
| No | Yes | ||
| N = 20,202 | N = 20,202 | ||
| Age, years | |||
| ≤49 | 7760 (38.4) | 7608 (37.7) | 0.02 |
| 50–64 | 7957 (39.4) | 8790 (43.5) | 0.08 |
| 65+ | 4485 (22.2) | 3804 (18.8) | 0.08 |
| Median ± (IQR) | 53.6 (45.1–63.5) | 53.3 (46.0–61.8) | 0.02 |
| Sex | |||
| Female | 8959 (44.4) | 8921 (44.2) | 0.004 |
| Male | 11,243 (55.7) | 11,281 (55.8) | 0.004 |
| Comorbidity | |||
| Hypertension | 13,677 (67.7) | 13,255 (65.6) | 0.04 |
| Diabetes | 3644 (18.0) | 3844 (19.0) | 0.03 |
| Rheumatoid disease | 55 (0.27) | 43 (0.21) | 0.01 |
| Alcohol-related disease | 1828 (9.05) | 1786 (8.84) | 0.01 |
| Asthma | 1766 (8.74) | 1780 (8.81) | 0.81 |
| Transplantation | 24 (0.12) | 20 (0.10) | 0.01 |
| Chronic liver disease | 6056 (30.0) | 6266 (31.0) | 0.02 |
| CKD or ESRD | 1314 (6.50) | 1398 (6.92) | 0.02 |
| COPD | 2627 (13.0) | 2662 (13.2) | 0.01 |
| HIV | 12 (0.06) | 16 (0.08) | 0.01 |
| Cancer | 1297 (6.42) | 1317 (6.52) | 0.004 |
| CHF | 1179 (5.84) | 1192 (5.90) | 0.003 |
| Stroke | 1700 (8.42) | 2014 (9.97) | 0.001 |
| Medications | |||
| Prednisolone | 14,248 (70.5) | 14,171 (70.2) | 0.008 |
| Mycophenolate mofetil | 27 (0.13) | 25 (0.12) | 0.003 |
| Cyclosporine | 43 (0.21) | 43 (0.21) | 0.000 |
| Tacrolimus | 21 (0.10) | 20 (0.10) | 0.002 |
| Azathioprine | 73 (0.36) | 71 (0.35) | 0.002 |
| Thiazides | 9122 (45.2) | 9075 (44.9) | 0.005 |
| ACEI | 8923 (44.2) | 8837 (43.7) | 0.009 |
| ARB | 8370 (41.4) | 8540 (42.3) | 0.017 |
| Propensity Score Matched | ||
|---|---|---|
| Statin | ||
| No | Yes | |
| Variables | (N = 20,202) | (N = 20,202) |
| Person-years | 104,665 | 141,459 |
| Follow-up time (y), Median ± (IQR) | 4.41 (2.01–7.89) | 6.90 (3.79–10.1) |
| Viral infection | ||
| Event | 7125 | 3723 |
| Rate # | 68.1 | 26.3 |
| Crude HR (95% CI) | 1 (Reference) | 0.41 (0.39, 0.42) *** |
| Adjusted HR † (95% CI) | 1 (Reference) | 0.40 (0.38, 0.41) *** |
| Hospitalization due to viral infection | ||
| Event | 76 | 38 |
| Rate # | 0.73 | 0.27 |
| Crude HR (95% CI) | 1 (Reference) | 0.38 (0.26, 0.56) *** |
| Adjusted HR † (95% CI) | 1 (Reference) | 0.37 (0.25, 0.55) *** |
| Intubation due to viral infection | ||
| Event | 3 | 3 |
| Rate # | 0.03 | 0.02 |
| Crude HR (95% CI) | 1 (Reference) | 0.72 (0.14, 3.60) |
| Adjusted HR † (95% CI) | 1 (Reference) | 0.39 (0.06, 2.41) |
| Statin | ||||||
|---|---|---|---|---|---|---|
| No (N = 20,202) | Yes (N = 20,202) | |||||
| Variables | Event | Rate # | Event | Rate # | Crude HR (95% CI) | Adjusted HR † (95% CI) |
| Age, years | ||||||
| ≤49 | 2903 | 68.8 | 1421 | 25.1 | 0.38 (0.36, 0.41) *** | 0.38 (0.36, 0.40) *** |
| 50–64 | 2816 | 70.0 | 1645 | 27.6 | 0.41 (0.39, 0.44) *** | 0.40 (0.38, 0.43) *** |
| 65+ | 1406 | 63.1 | 657 | 26.0 | 0.44 (0.40, 0.48) *** | 0.43 (0.39, 0.47) *** |
| Sex | ||||||
| Female | 3680 | 82.0 | 1741 | 27.7 | 0.36 (0.34, 0.38) *** | 0.36 (0.34, 0.38) *** |
| Male | 3445 | 57.6 | 1982 | 25.3 | 0.46 (0.43, 0.48) *** | 0.44 (0.42, 0.47) *** |
| Comorbidity § | ||||||
| No | 1332 | 97.1 | 580 | 31.5 | 0.35 (0.31, 0.38) *** | 0.35 (0.31, 0.38) *** |
| Yes | 5793 | 63.7 | 3143 | 25.6 | 0.42 (0.40, 0.44) *** | 0.41 (0.39, 0.43) *** |
| Medication Exposed | N | Event | Person-Year | Rate | Adjusted HR (95% CI) † |
|---|---|---|---|---|---|
| Statin # | |||||
| Non-statin | 20,202 | 7125 | 104,665 | 68.1 | 1.00 |
| ≤110 days | 3968 | 1250 | 23,638 | 52.9 | 0.73 (0.68, 0.77) *** |
| 111–350 days | 6059 | 1288 | 37,958 | 33.9 | 0.48 (0.45, 0.51) *** |
| 350–950 days | 5246 | 811 | 36,122 | 22.5 | 0.34 (0.32, 0.37) *** |
| >950 days | 4929 | 374 | 43,740 | 8.55 | 0.14 (0.13, 0.16) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.-R.; Chen, D.-H.; Liao, W.-C.; Ho, W.-C.; Yin, M.-C.; Lin, C.-L.; Chou, C.-H.; Peng, Y.-H. Statin Therapy and the Risk of Viral Infection: A Retrospective Population-Based Cohort Study. J. Clin. Med. 2022, 11, 5626. https://doi.org/10.3390/jcm11195626
Wu B-R, Chen D-H, Liao W-C, Ho W-C, Yin M-C, Lin C-L, Chou C-H, Peng Y-H. Statin Therapy and the Risk of Viral Infection: A Retrospective Population-Based Cohort Study. Journal of Clinical Medicine. 2022; 11(19):5626. https://doi.org/10.3390/jcm11195626
Chicago/Turabian StyleWu, Biing-Ru, Ding-Han Chen, Wei-Chih Liao, Wen-Chao Ho, Ming-Chien Yin, Cheng-Li Lin, Chia-Hui Chou, and Yi-Hao Peng. 2022. "Statin Therapy and the Risk of Viral Infection: A Retrospective Population-Based Cohort Study" Journal of Clinical Medicine 11, no. 19: 5626. https://doi.org/10.3390/jcm11195626
APA StyleWu, B.-R., Chen, D.-H., Liao, W.-C., Ho, W.-C., Yin, M.-C., Lin, C.-L., Chou, C.-H., & Peng, Y.-H. (2022). Statin Therapy and the Risk of Viral Infection: A Retrospective Population-Based Cohort Study. Journal of Clinical Medicine, 11(19), 5626. https://doi.org/10.3390/jcm11195626






