A Simple Review of Small Vessel Disease Manifestation in the Brain, Retina, and Kidneys
Abstract
1. Introduction
2. Associations between Chronic Kidney Disease and Cerebrovascular Disease in Clinical Studies
3. Associations between Cerebrovascular Disease and Retinal Changes in Population-Based Studies
4. Associations between Kidney Disease and Retina Vasculature Changes in Clinical Studies
5. Associations between CKD, Retina, and CVD in Basic Science Research
5.1. Embryology and Genetics
5.2. Cardiovascular Physiology
5.3. Pathology
6. Clinical Implications and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ito, S.; Nagasawa, T.; Abe, M.; Mori, T. Strain vessel hypothesis: A viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens. Res. 2009, 32, 115–121. [Google Scholar] [CrossRef]
- El Husseini, N.; Fonarow, G.C.; Smith, E.E.; Ju, C.; Sheng, S.; Schwamm, L.H.; Hernandez, A.F.; Schulte, P.J.; Xian, Y.; Goldstein, L.B. Association of Kidney Function With 30-Day and 1-Year Poststroke Mortality and Hospital Readmission. Stroke 2018, 49, 2896–2903. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.; Chang, K.-H.; Liao, H.-W.; Chang, S.-C.; Ovbiagele, B. Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ 2010, 341, c4249. [Google Scholar] [CrossRef]
- Molshatzki, N.; Orion, D.; Tsabari, R.; Schwammenthal, Y.; Merzeliak, O.; Toashi, M.; Tanne, D. Chronic Kidney Disease in Patients with Acute Intracerebral Hemorrhage: Association with Large Hematoma Volume and Poor Outcome. Cerebrovasc. Dis. 2010, 31, 271–277. [Google Scholar] [CrossRef]
- Molnar, A.O.; Bota, S.E.; Garg, A.X.; Harel, Z.; Lam, N.; McArthur, E.; Nesrallah, G.; Perl, J.; Sood, M.M. The risk of major hemorrhage with CKD. J. Am. Soc. Nephrol. 2016, 27, 2825–2832. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, N.W.; Yun, J.M.; Lee, J.E.; Lim, J.-S.; Cho, B.L.; Kwon, H.-M.; Park, J.-H. Kidney dysfunction and cerebral microbleeds in neurologically healthy adults. PLoS ONE 2017, 12, e0172210. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Wing, J.J.; Menon, R.S.; Burgess, R.E.; Gibbons, M.C.; Sobotka, I.; German, L.; Shara, N.M.; Fernandez, S.; Jayam-Trouth, A.; et al. Association of Chronic Kidney Disease with Cerebral Microbleeds in Patients with Primary Intracerebral Hemorrhage. Stroke 2013, 44, 2409–2413. [Google Scholar] [CrossRef]
- Cho, A.-H.; Lee, S.B.; Han, S.J.; Shon, Y.-M.; Yang, D.-W.; Kim, B.S. Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology 2009, 73, 1645–1648. [Google Scholar] [CrossRef]
- Akoudad, S.; Sedaghat, S.; Hofman, A.; Koudstaal, P.J.; Van Der Lugt, A.; Ikram, M.A.; Vernooij, M.W. Kidney Function and Cerebral Small Vessel Disease in the General Population. Int. J. Stroke 2015, 10, 603–608. [Google Scholar] [CrossRef]
- Kim, S.H.; Yun, J.M.; Jeong, S.-M.; Kim, S.; Yoo, T.G.; Lee, J.E.; Lim, J.-S.; Jeong, H.-Y.; Nam, K.-W.; Kwon, H.-M.; et al. Kidney Dysfunction Impact on White Matter Hyperintensity Volume in Neurologically Healthy Adults. Sci. Rep. 2019, 9, 8596. [Google Scholar] [CrossRef]
- Wada, M.; Nagasawa, H.; Iseki, C.; Takahashi, Y.; Sato, H.; Arawaka, S.; Kawanami, T.; Kurita, K.; Daimon, M.; Kato, T. Cerebral small vessel disease and chronic kidney disease (CKD): Results of a cross-sectional study in community-based Japanese elderly. J. Neurol. Sci. 2008, 272, 36–42. [Google Scholar] [CrossRef]
- Xiao, L.; Lan, W.; Sun, W.; Dai, Q.; Xiong, Y.; Li, L.; Zhou, Y.; Zheng, P.; Fan, W.; Ma, N.; et al. Chronic Kidney Disease in Patients with Lacunar Stroke: Association with Enlarged Perivascular Spaces and Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease. Stroke J. Cereb. Circ. 2015, 46, 2081–2086. [Google Scholar] [CrossRef]
- Xu, X.-H.; Ye, X.-H.; Cai, J.-S.; Gao, T.; Zhao, G.-H.; Zhang, W.-J.; Tong, L.-S.; Gao, F. Association of Renal Dysfunction with Remote Diffusion-Weighted Imaging Lesions and Total Burden of Cerebral Small Vessel Disease in Patients with Primary Intracerebral Hemorrhage. Front. Aging Neurosci. 2018, 10, 171. [Google Scholar] [CrossRef]
- Seliger, S.L.; Weiner, D.E. Cognitive Impairment in Dialysis Patients: Focus on the Blood Vessels? Am. J. Kidney Dis. 2013, 61, 187–190. [Google Scholar] [CrossRef][Green Version]
- Lau, W.L.; Huisa, B.N.; Fisher, M. The Cerebrovascular-Chronic Kidney Disease Connection: Perspectives and Mechanisms. Transl. Stroke Res. 2016, 8, 67–76. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Couper, D.; Klein, B.E.K.; Liao, D.-P.; Hubbard, L.D.; Mosley, T.H.; For the ARIC Investigators. Cerebral White Matter Lesions, Retinopathy, and Incident Clinical Stroke. JAMA 2002, 288, 67–74. [Google Scholar] [CrossRef]
- Cooper, L.S.; Wong, T.Y.; Klein, R.; Sharrett, A.R.; Bryan, R.N.; Hubbard, L.D.; Couper, D.; Heiss, G.; Sorlie, P.D. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: The atherosclerosis risk in communities study. Stroke 2006, 37, 82–86. [Google Scholar] [CrossRef]
- Wong, T.; Mitchell, P. The eye in hypertension. Lancet 2007, 369, 425–435. [Google Scholar] [CrossRef]
- Longstreth, W.; Larsen, E.K.M.; Klein, R.; Wong, T.Y.; Sharrett, A.R.; Lefkowitz, D.; Manolio, T.A. Associations between Findings on Cranial Magnetic Resonance Imaging and Retinal Photography in the Elderly: The Cardiovascular Health Study. Am. J. Epidemiol. 2006, 165, 78–84. [Google Scholar] [CrossRef][Green Version]
- Wong, T.Y.; Kamineni, A.; Klein, R.; Sharrett, A.R.; Klein, B.E.; Siscovick, D.S.; Cushman, M.; Duncan, B.B. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons. Arch. Intern. Med. 2006, 166, 2388–2394. [Google Scholar] [CrossRef]
- Ikram, M.K.; De Jong, F.J.; Van Dijk, E.J.; Prins, N.; Hofman, A.; Breteler, M.M.; De Jong, P.T.V.M. Retinal vessel diameters and cerebral small vessel disease: The Rotterdam Scan Study. Brain 2005, 129, 182–188. [Google Scholar] [CrossRef]
- Mitchell, P.; Wang, J.J.; Wong, T.Y.; Smith, W.; Klein, R.; Leeder, S.R. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology 2005, 65, 1005–1009. [Google Scholar] [CrossRef]
- Ooi, Q.L.; Tow, F.K.N.-F.H.; Deva, R.; Alias, M.A.; Kawasaki, R.; Wong, T.Y.; Mohamad, N.; Colville, D.; Hutchinson, A.; Savige, J. The Microvasculature in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1872–1878. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Pistilli, M.; Ying, G.-S.; Daniel, E.; Maguire, M.; Xie, D.; Roy, J.; Whittock-Martin, R.; Ostroff, C.P.; Lo, J.C.; et al. Association Between Progression of Retinopathy and Concurrent Progression of Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. JAMA Ophthalmol. 2019, 137, 767–774. [Google Scholar] [CrossRef]
- Deva, R.; Alias, M.A.; Colville, D.; Tow, F.K.N.-F.H.; Ooi, Q.L.; Chew, S.; Mohamad, N.; Hutchinson, A.F.; Koukouras, I.; Power, D.A.; et al. Vision-Threatening Retinal Abnormalities in Chronic Kidney Disease Stages 3 to 5. Clin. J. Am. Soc. Nephrol. 2011, 6, 1866–1871. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, L.; Pan, Y.; Yang, S.; Zhang, L.; Wang, H. Ocular fundus pathology and chronic kidney disease in a Chinese population. BMC Nephrol. 2011, 12, 62. [Google Scholar] [CrossRef]
- Choi, J.; Moon, J.W.; Shin, H.J. Chronic Kidney Disease, Early Age-related Macular Degeneration, and Peripheral Retinal Drusen. Ophthalmic Epidemiol. 2011, 18, 259–263. [Google Scholar] [CrossRef]
- Liew, G.; Mitchell, P.; Wong, T.Y.; Iyengar, S.K.; Wang, J.J. CKD Increases the Risk of Age-Related Macular Degeneration. J. Am. Soc. Nephrol. 2008, 19, 806–811. [Google Scholar] [CrossRef]
- Balmforth, C.; Van Bragt, J.J.; Ruijs, T.; Cameron, J.R.; Kimmitt, R.; Moorhouse, R.; Czopek, A.; Hu, M.K.; Gallacher, P.J.; Dear, J.W.; et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 2016, 1, e89173. [Google Scholar] [CrossRef]
- Liew, G.; Mitchell, P.; Wong, T.; Wang, J. Retinal Microvascular Signs Are Associated with Chronic Kidney Disease in Persons with and without Diabetes. Kidney Blood Press. Res. 2012, 35, 589–594. [Google Scholar] [CrossRef]
- Awua-Larbi, S.; Wong, T.Y.; Cotch, M.F.; Durazo-Arvizu, R.; Jacobs, D.R.; Klein, B.E.K.; Klein, R.; Lima, J.; Liu, K.; Kramer, H. Retinal arteriolar caliber and urine albumin excretion: The Multi-Ethnic Study of Atherosclerosis. Nephrol. Dial. Transplant. 2011, 26, 3523–3528. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Shankar, A.; Koh, D.; Chia, K.S.; Saw, S.M.; Lim, S.C.; Tai, E.S.; Wong, T.Y. Retinal Microvascular Caliber and Chronic Kidney Disease in an Asian Population. Am. J. Epidemiol. 2008, 169, 625–632. [Google Scholar] [CrossRef]
- Wong, T.Y.; Coresh, J.; Klein, R.; Muntner, P.; Couper, D.J.; Sharrett, A.R.; Klein, B.E.K.; Heiss, G.; Hubbard, L.D.; Duncan, B.B. Retinal Microvascular Abnormalities and Renal Dysfunction: The Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 2004, 15, 2469–2476. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; MacGillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef]
- Hughes, S.; Yang, H.; Chan-Ling, T. Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1217–1228. [Google Scholar]
- Bertulli, L.; Robert, T. Embryological development of the human cranio-facial arterial system: A pictorial review. Surg. Radiol. Anat. 2021, 43, 961–973. [Google Scholar] [CrossRef]
- Menshawy, K.; Mohr, J.P.; Gutierrez, J.G.A. A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J. Stroke 2015, 17, 144–158. [Google Scholar] [CrossRef]
- Yu, J.; Xu, N.; Zhao, Y.; Yu, J. Clinical importance of the anterior choroidal artery: A review of the literature. Int. J. Med Sci. 2018, 15, 368–375. [Google Scholar] [CrossRef]
- Kathuria, S.; Gregg, L.; Chen, J.; Gandhi, D. Normal Cerebral Arterial Development and Variations. Semin. Ultrasound CT MRI 2011, 32, 242–251. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; D’Amore, P.A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004, 48, 1045–1058. [Google Scholar] [CrossRef]
- Lutty, G.A.; McLeod, D.S. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog. Retin. Eye Res. 2017, 62, 58–76. [Google Scholar] [CrossRef]
- Stone, J.; Chan-Ling, T.; Pe’Er, J.; Itin, A.; Gnessin, H.; Keshet, E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 1996, 37, 290–299. [Google Scholar]
- Gregg, L.; Millán, D.S.; Orru’, E.; Tamargo, R.J.; Gailloud, P. Ventral and Dorsal Persistent Primitive Ophthalmic Arteries. Oper. Neurosurg. 2016, 12, 141–152. [Google Scholar] [CrossRef]
- Zhao, S.; Overbeek, P. Regulation of choroid development by the retinal pigment epithelium. Mol. Vis. 2001, 7, 277–282. [Google Scholar]
- Sakamoto, T.; Sakamoto, H.; Murphy, T.L.; Spee, C.; Soriano, D.; Ishibashi, T.; Hinton, D.R.; Ryan, S.J. Vessel Formation by Choroidal Endothelial Cells In Vitro Is Modulated by Retinal Pigment Epithelial Cells. Arch. Ophthalmol. 1995, 113, 512–520. [Google Scholar] [CrossRef]
- Rousseau, B. Involvement of fibroblast growth factors in choroidal angiogenesis and retinal vascularization. Exp. Eye Res. 2003, 77, 147–156. [Google Scholar] [CrossRef]
- Gogat, K.; Le Gat, L.; Berghe, L.V.D.; Marchant, D.; Kobetz, A.; Gadin, S.; Gasser, B.; Quere, I.; Abitbol, M.; Menasche, M. VEGF and KDR Gene Expression during Human Embryonic and Fetal Eye Development. Investig. Opthalmol. Vis. Sci. 2004, 45, 7–14. [Google Scholar] [CrossRef]
- Mauer, S.M.; Barbosa, J.; Vernier, R.L.; Kjellstrand, C.M.; Buselmeier, T.J.; Simmons, R.L.; Najarian, J.S.; Goetz, F.C. Development of Diabetic Vascular Lesions in Normal Kidneys Transplanted into Patients with Diabetes Mellitus. N. Engl. J. Med. 1976, 295, 916–920. [Google Scholar] [CrossRef]
- Di Donato, I.; Bianchi, S.; De Stefano, N.; Dichgans, M.; Dotti, M.T.; Duering, M.; Jouvent, E.; Korczyn, A.D.; Lesnik-Oberstein, S.A.J.; Malandrini, A.; et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: Update on clinical, diagnostic, and management aspects. BMC Med. 2017, 15, 41. [Google Scholar] [CrossRef]
- Goldstein, E.D.; Shakoor, A.; Majersik, J.J. Branch Retinal Vein Occlusion and Venous Abnormalities in CADASIL. Neurologist 2020, 25, 178–179. [Google Scholar] [CrossRef]
- Kwa, V.I.; Van Der Sande, J.J.; Stam, J.; Tijmes, N.; Vrooland, J.L. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology 2002, 59, 1536–1540. [Google Scholar] [CrossRef]
- Haffner, C.; Malik, R.; Dichgans, M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J. Cereb. Blood Flow Metab. 2015, 36, 158–171. [Google Scholar] [CrossRef]
- Lu, D.; Kassab, G.S. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 2011, 8, 1379–1385. [Google Scholar] [CrossRef]
- Mogi, M.; Horiuchi, M. Clinical Interaction between Brain and Kidney in Small Vessel Disease. Cardiol. Res. Pract. 2011, 2011, 306189. [Google Scholar] [CrossRef]
- Kelly, D.; Rothwell, P.M. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J. Neurol. Neurosurg. Psychiatry 2019, 91, 88–97. [Google Scholar] [CrossRef]
- Bradbury, M.W.B.; Lightman, S.L. The blood-brain interface. Eye 1990, 4, 249–254. [Google Scholar] [CrossRef]
- Campbell, M.; Humphries, P. The blood-retina barrier tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2013, 763, 70–84. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. The Blood–Retinal Barrier in Retinal Disease. Eur. Ophthalmic Rev. 2009, 3, 105. [Google Scholar] [CrossRef]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Farrah, T.E.; Dhillon, B.; Keane, P.A.; Webb, D.J.; Dhaun, N. The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons. Kidney Int. 2020, 98, 323–342. [Google Scholar] [CrossRef]
- Pollak, M.R.; Quaggin, S.E.; Hoenig, M.P.; Dworkin, L.D. The Glomerulus: The Sphere of Influence. Clin. J. Am. Soc. Nephrol. 2014, 9, 1461–1469. [Google Scholar] [CrossRef]
- Boutaud, A.; Borza, D.-B.; Bondar, O.; Gunwar, S.; Netzer, K.-O.; Singh, N.; Ninomiya, Y.; Sado, Y.; Noelken, M.E.; Hudson, B.G. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the non-collagenous NC1 domains. J. Biol. Chem. 2000, 275, 30716–30724. [Google Scholar] [CrossRef]
- Savige, J.; Sheth, S.; Leys, A.; Nicholson, A.; Mack, H.G.; Colville, D. Ocular Features in Alport Syndrome: Pathogenesis and Clinical Significance. Clin. J. Am. Soc. Nephrol. 2015, 10, 703–709. [Google Scholar] [CrossRef]
- Colville, D.; Savige, J.; Branley, P.; Wilson, D. Ocular abnormalities in thin basement membrane disease. Br. J. Ophthalmol. 1997, 81, 373–377. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Fervenza, F.C.; Sethi, S.; Pulido, J.S. Manifestations of Complement-Mediated and Immune Complex-Mediated Membranoproliferative Glomerulonephritis. Ophthalmology 2016, 123, 1588–1594. [Google Scholar] [CrossRef]
- Whitmore, S.S.; Sohn, E.H.; Chirco, K.R.; Drack, A.V.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Complement activation and choriocapillaris loss in early AMD: Implications for pathophysiology and therapy. Prog. Retin. Eye Res. 2014, 45, 1–29. [Google Scholar] [CrossRef]
- Aronov, M.; Allon, R.; Stave, D.; Belkin, M.; Margalit, E.; Fabian, I.; Rosenzweig, B. Retinal Vascular Signs as Screening and Prognostic Factors for Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Current Evidence. J. Pers. Med. 2021, 11, 665. [Google Scholar] [CrossRef]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Klein, B.E.K.; Wang, J.J.; Mitchell, P.; Vingerling, J.R.; De Jong, P.T.V.M.; et al. Prediction of Incident Stroke Events Based on Retinal Vessel Caliber: A Systematic Review and Individual-Participant Meta-Analysis. Am. J. Epidemiol. 2009, 170, 1323–1332. [Google Scholar] [CrossRef]
- Allon, R.; Aronov, M.; Belkin, M.; Maor, E.; Shechter, M.; Fabian, I.D. Retinal Microvascular Signs as Screening and Prognostic Factors for Cardiac Disease: A Systematic Review of Current Evidence. Am. J. Med. 2021, 134, 36–47.e7. [Google Scholar] [CrossRef]
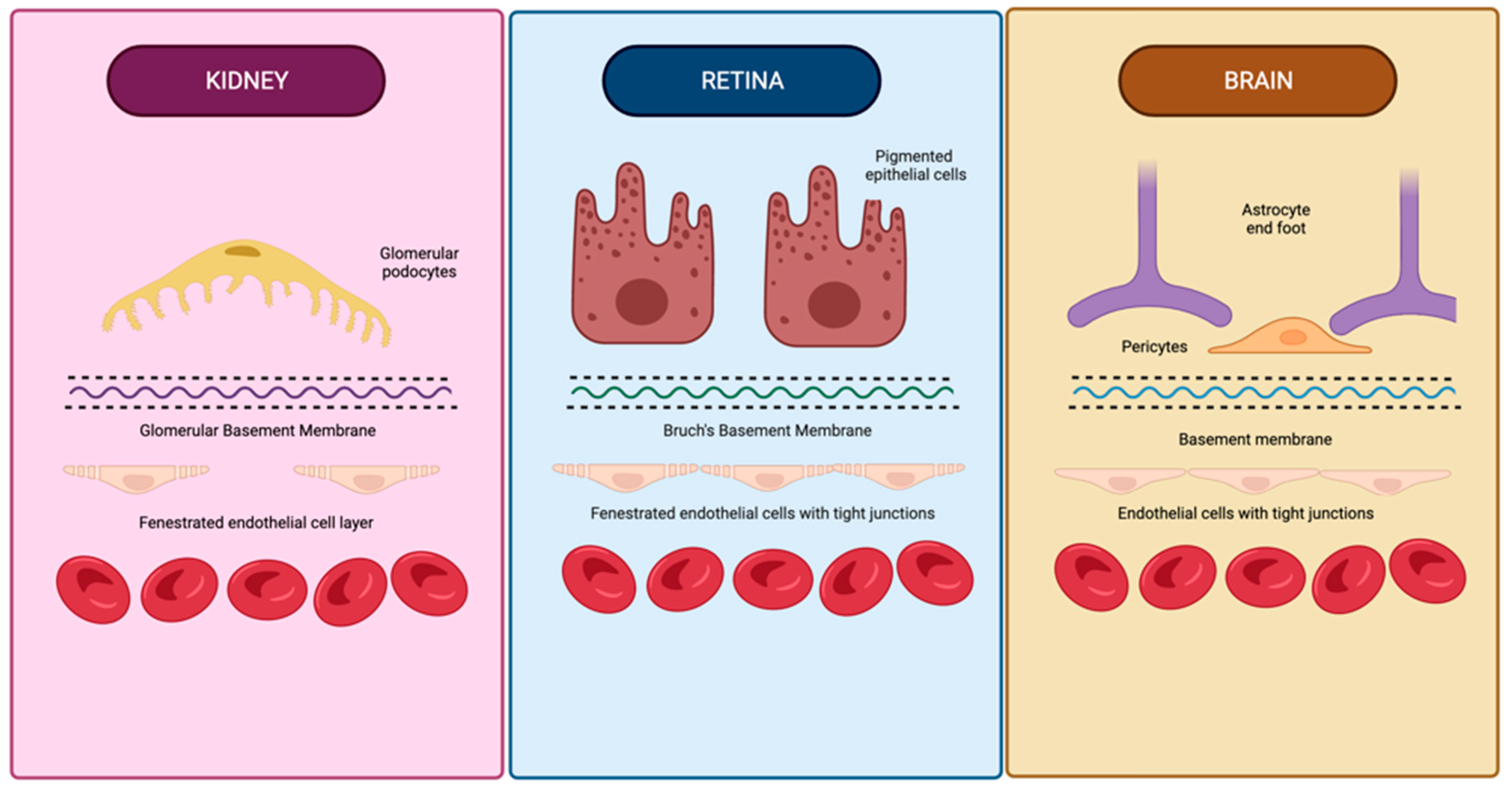
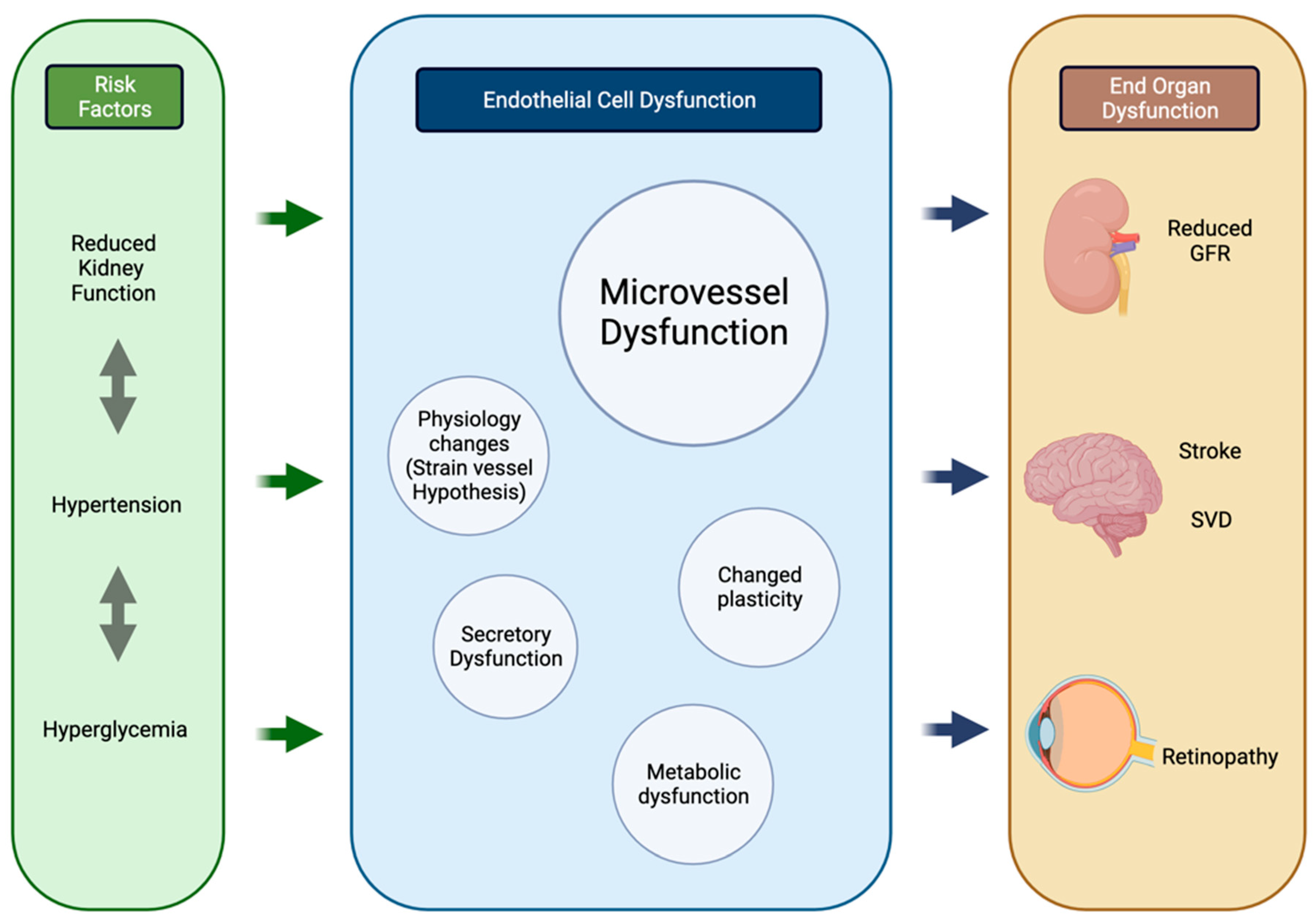
| Outcome | Study | Type | Sample Size | Demographics | Conclusion |
|---|---|---|---|---|---|
| Stroke/hemorrhage and GFR | Ell Husseini et al. (2018) [2] | Prospective cohort | 204,652 | Age: >65 years | Within a year after hospitalization for ischemic stroke, GFR and dialysis status on admission are associated with post-stroke mortality (HR 2.09, 95% CI 1.66–2.63) and hospital readmissions (HR 2.55, 95% CI 2.44–2.66). |
| Lee et al. (2010) [3] | Meta-analysis | 284,672 | Varying | A baseline GFR <60 mL/min/1.73 m2 was independently related to incident stroke (RR 1.43, 95% CI 1.31 to 1.57; p < 0.001). | |
| Molshatzki et al. (2011) [4] | Prospective cohort | 128 | Avg age: 71.7 years | Patients with moderate/severe CKD (GFR <45) had >4-fold adjusted hazard ratio for mortality over 1 year (4.29; 95% CI = 1.69–10.90) and a 2.3-fold higher hematoma volume (p = 0.04) compared to patients with no impairment. | |
| Molnar et al. (2016) [5] | Retrospective cohort | 516,197 | Age: ≥40 years | Incidence of hemorrhage increased 20-fold across declining eGFR and increasing urine ACR groupings (highest eGFR/lowest ACR: 0.5%; lowest eGFR/highest ACR: 10.1%). | |
| CMBs and GFR | Kim et al. (2017) [6] | Cross-sectional | 2518 | Age: 40–79 years without symptomatic stroke history | Subjects with CMB demonstrated a higher proportion of moderate-to-severe renal dysfunction than those without CMB (15.5% vs. 5.0%, p < 0.001). |
| Ovbiagele et al. (2013) [7] | Retrospective/prospective cohort | 197 | Age: ≥18 years with primary ICH | CKD was associated with the presence of CMB (adjusted OR, 2.70; 95% CI, 1.10–6.59) and number of CMB (adjusted RR, 2.04; 95% CI, 1.27–3.27. | |
| Cho et al. (2009) [8] | Retrospective cohort | 142 | Age: Avg 66.7 years | Low GFR levels were associated with the presence of cerebral microbleeds (OR, 3.85; 95% CI, 1.52 to 9.76, p = 0.004). | |
| WMHs and kidney function | Akoudad et al. (2015) [9] | Prospective cohort | 2526 | Age: ≥45 years | Worse kidney function was consistently associated with a larger white matter lesion volume (mean difference per standard deviation increase in albumin-to-creatinine ratio: 0.09, 95% CI 0.05; 0.12; per standard deviation decrease in creatinine-based GFR: −0.04, 95% CI −0.08;−0.01). |
| Kim et al. (2019) [10] | Cross-sectional | 2203 | Age: ≥40 years | Subjects with both significant albuminuria and GFR < 60 had a significantly higher WMH volume (β = 0.652; p < 0.001). | |
| Wada et al. (2008) [11] | Cross-sectional | 625 | Age: 61–72 years | Subjects with lower GFR levels tended to have more lacunar infarcts and higher grades of WMHs. In addition, the mean grades of WMHs or the mean number of lacunar infarcts in the subjects with albuminuria were greater than those in subjects without albuminuria. | |
| Xiao et al. (2015) [12] | Cross-sectional | 413 | Age: Avg 64 years | Proteinuria and impaired eGFR were correlated with the severity of EPVS in both centrum semiovale (OR 2.59; 95% CI 1.19–5.64 and OR 2.37; 95% CI 1.19–4.73) and basal ganglia (OR 5.12; 95% CI 2.70–12.10 and OR 4.17; 95% CI 2.08–8.37). |
| Outcome | Study | Type | Sample Size | Demographics | Conclusion |
|---|---|---|---|---|---|
| White Matter Disease and retinopathy | Wong et al. (2002) [16] | Prospective cohort | 1684 | Age: 51–72 years | Persons with retinopathy were more likely to have WMLs than those without retinopathy (22.9% vs. 9.9%; OR, 2.5; 95% CI: 1.5–4.0). |
| Stroke and retinopathy | Wong et al. (2002) [16] | Cross-sectional | 2050 | Age: 69–97 yearsWithout diabetes | Retinopathy was found to be associated with prevalent stroke (OR 2.0). |
| Cooper et al. (2005) [17] | Cross-sectional | 1684 | Age: 55–74 yearswithout a history of clinical stroke | Cerebral infarcts were found associated with soft exudates (OR 2.08; 95% CI: 0.69–6.31). | |
| Mitchell et al. (2005) [18] | Prospective cohort | 3654 | Age: >49 years | Retinopathy was significantly associated with combined stroke events (RR 1.7; 95% CI: 1.0–2.8) in persons without diabetes. | |
| Stroke and retinal vascular changes | Longstreth et al. (2006) [19] | Case-control | 1717 | Age: >65 years | Smaller arteriovenous ratio (per standard deviation decrease) was found associated with prevalent infarcts (OR 1.18; 95% CI: 1.05–1.34; p = 0.007). Arteriovenous nicking was found associated with prevalent (OR 1.84; 95% CI, 1.23, 2.76; p = 0.003) and incident (OR 1.84; 95% CI: 1.15–2.94; p = 0.011) infarcts. |
| Wong et al. (2006) [20] | Prospective cohort | 1992 | Age: 69–97 years | Larger retinal venular caliber was associated with incident stroke (OR 2.2; 95% CI: 1.1–4.3). | |
| Ikram et al. (2006) [21] | Prospective cohort | 5540 | Age: >55 Without history of stroke | Larger venular diameters were associated with an increased risk of stroke (HR per SD increase 1.12; 95% CI: 1.02–1.24) and cerebral infarction (HR: 1.15; 95% CI: 1.02–1.29). | |
| Mitchell et al. (2005) [22] | Prospective cohort | 3654 | Age: >49 years | Combined stroke events were more frequent in participants with retinopathy (5.7%), with moderate/severe arteriovenous nicking (4.2%), or with focal arteriolar narrowing (7.2%) compared with those without (1.9%). |
| Outcome | Study | Type | Sample Size | Demographics | Conclusion |
|---|---|---|---|---|---|
| CKD and retinopathy | Grunwald et al. (2019) [24] | Case-Control | 1025 | Participants with CKD | CKD progression associated with worsening of retinopathy in comparison with participants with stable retinopathy (OR 2.24; 95% CI: 1.28–3.91). |
| Deva et al. (2011) [25] | Case-Control | 150 | Participants with CKD stage 3–5 Australia | CKD stages 3 to 5 (OR 1.79; CI: 1.00–3.20) were independent determinants of macular degeneration. | |
| Gao et al. (2011) [26] | Cross-sectional | 9670 | Participants with CKD | Prevalence of retinopathy is higher in CKD patients than participants without CKD (28.5% vs. 16.3%, p < 0.001). | |
| Choi et al. (2011) [27] | Cross-sectional | 3008 | Age: 50–87 Participants with CKD South Korea | Participants with CKD are more likely to develop early age-related macular degeneration (OR 1.68; 95% CI: 1.04–2.72) and peripheral retinal drusen (OR 2.01; 95% CI: 1.02–3.99) than those without. | |
| Liew et al. (2008) [28] | Case-Control | 1183 | Age: >54 Participants with CKD Australia | Individuals with moderate CKD were three times more likely to develop early AMD than individuals with no/mild CKD (OR 3.2; 95% CI: 1.8–5.7). | |
| CKD and retinal vascular/Structural changes | Balmforth et al. (2016) [29] | Prospective cross-sectional | 150 | 50 patients with hypertension (clinic BP greater than or equal to 140/90 mmHg prior any treatment) 50 with CKD 50 matched healthy controls | Retinal thickness, macular volume, and choroidal thickness were all reduced in CKD compared with hypertensive and healthy subjects. |
| Liew et al. (2012) [30] | Cross-sectional | 2971 | Age: >49 | CKD was associated with both presence of retinopathy (OR, 1.2, 95% CI: 1.0–1.5) and venular dilation (OR 1.2, 95% CI: 1.0–1.5). | |
| Awua-Larbi et al. (2011) [31] | Case-Control Study | 675 | Age: 45–84 Without baseline clinical cardiovascular disease | Both narrower CRAE (OR 1.55; 95% CI: 1.17–2.04) and wider CRAE (OR 1.44; 95% CI: 1.07–1.93) were significantly associated with albuminuria. | |
| Sabanayagam et al. (2009) [32] | Cross-sectional study | 2380 | Age: 40–80 Singapore | CRAE was associated with CKD, (OR 1.42; 95% CI: 1.03–1.96) for eGFR < 60 and 1.80 (1.11–1.96) for micro/macroalbuminuria. | |
| Wong et al. (2004) [33] | Prospective study | 10,056 | Age: 45–64 | Individuals with retinopathy (OR 2.0; 95% CI: 1.4–2.8), microaneurysms (OR, 2.0; 95% CI: 1.3–3.1), retinal hemorrhages (OR, 2.6; 95% CI: 1.6–4.0), soft exudates (OR, 2.7; 95% CI: 1.6–4.8), and arteriovenous nicking (OR, 1.4; 95% CI: 1.0–1.9) were more likely to develop renal dysfunction than individuals without these abnormalities. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, K.; Lu, Y.; Bavishi, S.; Mishra, N.; TomThundyil, S.; Sawant, S.A.; Shahjouei, S.; Abedi, V.; Zand, R. A Simple Review of Small Vessel Disease Manifestation in the Brain, Retina, and Kidneys. J. Clin. Med. 2022, 11, 5546. https://doi.org/10.3390/jcm11195546
Abbas K, Lu Y, Bavishi S, Mishra N, TomThundyil S, Sawant SA, Shahjouei S, Abedi V, Zand R. A Simple Review of Small Vessel Disease Manifestation in the Brain, Retina, and Kidneys. Journal of Clinical Medicine. 2022; 11(19):5546. https://doi.org/10.3390/jcm11195546
Chicago/Turabian StyleAbbas, Kinza, Yezhong Lu, Shreya Bavishi, Nandini Mishra, Saumya TomThundyil, Shreeya Atul Sawant, Shima Shahjouei, Vida Abedi, and Ramin Zand. 2022. "A Simple Review of Small Vessel Disease Manifestation in the Brain, Retina, and Kidneys" Journal of Clinical Medicine 11, no. 19: 5546. https://doi.org/10.3390/jcm11195546
APA StyleAbbas, K., Lu, Y., Bavishi, S., Mishra, N., TomThundyil, S., Sawant, S. A., Shahjouei, S., Abedi, V., & Zand, R. (2022). A Simple Review of Small Vessel Disease Manifestation in the Brain, Retina, and Kidneys. Journal of Clinical Medicine, 11(19), 5546. https://doi.org/10.3390/jcm11195546








