Visualization and Grading of Vitreous Floaters Using Dynamic Ultra-Widefield Infrared Confocal Scanning Laser Ophthalmoscopy: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
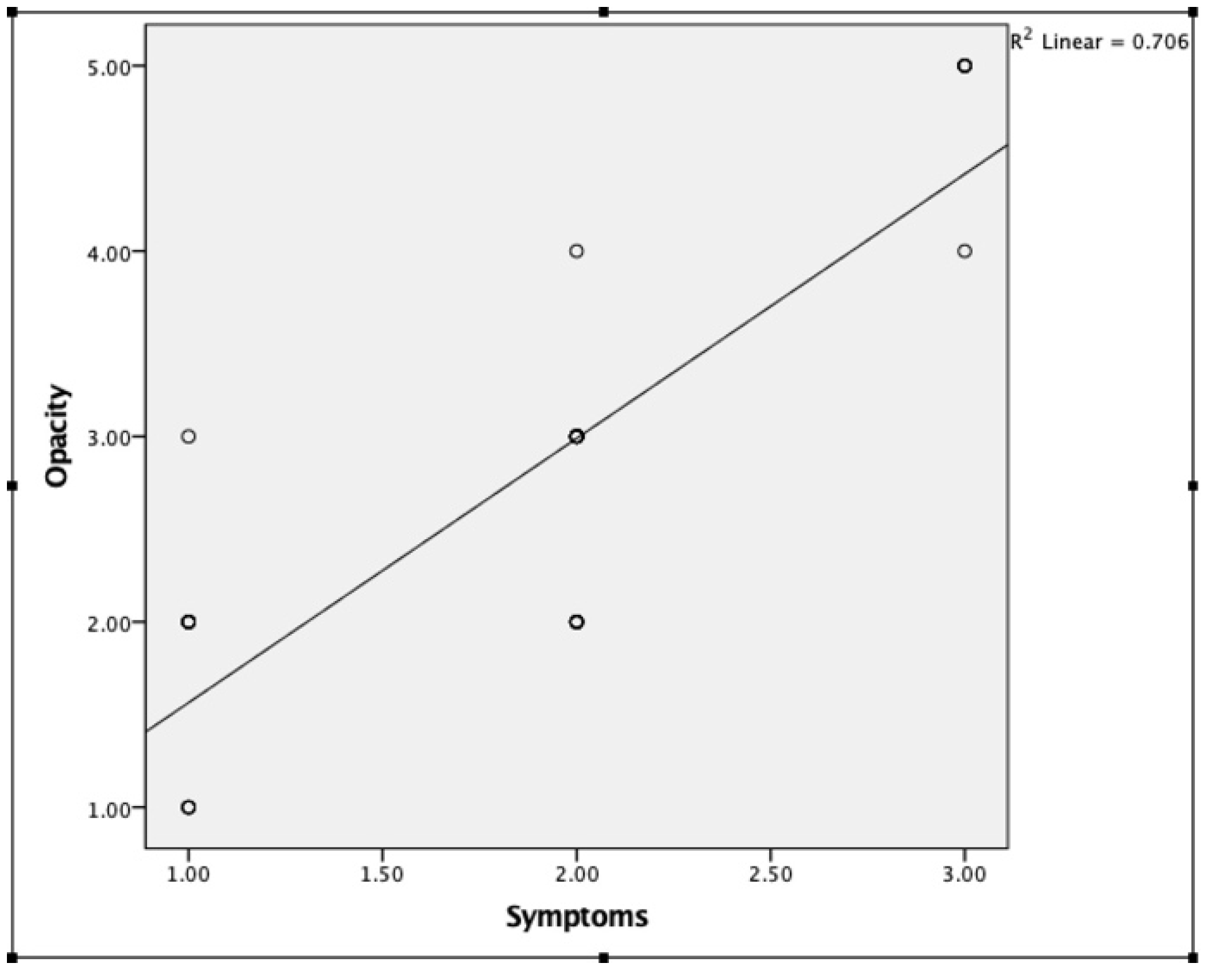
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, B.F.; Webb, J.R.; Schroeder, M.C.; North, C.S. Prevalence of vitreous floaters in a community sample of smartphone users. Int. J. Ophthalmol. 2013, 6, 402–405. [Google Scholar] [PubMed]
- Tozer, K.; Johnson, M.; Sebag, J. Vitreous Aging and Posterior Vitreous Detachment. In Vitreous Health and Disease; Springer: Manhattan, NY, USA, 2014; pp. 131–150. [Google Scholar]
- Sebag, J. Age-related changes in human vitreous structure. Graefe’s Arch. Clin. Exp. Ophthalmol. 1987, 225, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Milston, R.; Madigan, M.C.; Sebag, J. Vitreous floaters: Etiology, diagnostics, and management. Surv. Ophthalmol. 2016, 61, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Oksala, A. Ultrasonic findings in the vitreous body at various ages. Albrecht Von Graefes Arch. Für Klin. Und Exp. Ophthalmol. 1978, 207, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Imaging vitreous. Eye 2002, 16, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Ketterling, J.; Mamou, J.; Lloyd, H.O.; Filoux, E.; Coleman, D.J. Pulse-Encoded Ultrasound Imaging of the Vitreous with an Annular Array. Ophthalmic Surgery, Lasers Imaging Retin. 2012, 43, 82–86. [Google Scholar] [CrossRef]
- Salcedo-Villanueva, G.; Trujillo-Alvarez, M.; Becerra-Revollo, C.; Ibarra-Elizalde, E.; Mayorquín-Ruiz, M.; Velez-Montoya, R.; García-Aguirre, G.; Gonzalez-Salinas, R.; Morales-Cantón, V.; Quiroz-Mercado, H.; et al. A Proposed Method to Quantify Vitreous Hemorrhage by Ultrasound. Clin. Ophthalmol. 2019, 13, 2377–2384. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Flynn, H.W.; Fisher, Y.L. “Floater Scotoma” Demonstrated on Spectral-Domain Optical Coherence Tomography and Caused by Vitreous Opacification. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 415–418. [Google Scholar] [CrossRef]
- Son, G.; Sohn, J.; Kong, M. Acute symptomatic vitreous floaters assessed with ultra-wide field scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Ruminski, D.; Sebag, J.; Toledo, R.D.; Jiménez-Villar, A.; Nowak, J.K.; Manzanera, S.; Artal, P.; Grulkowski, I. Volumetric Optical Imaging and Quantitative Analysis of Age-Related Changes in Anterior Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2021, 62, 31. [Google Scholar] [CrossRef]
- Mainster, M.A.; Timberlake, G.T.; Webb, R.H.; Hughes, G.W. Scanning laser ophthalmoscopy—Clinical applications. Ophthalmology 1982, 89, 852–857. [Google Scholar] [CrossRef]
- Kakehashi, A.; Ishiko, S.; Konno, S.; Akiba, J.; Kado, M.; Yoshida, A. Observing the posterior vitreous by means of the scanning laser ophthalmoscope. Arch. Ophthalmol. 1995, 113, 558–560. [Google Scholar] [CrossRef]
- Mayne, R.; Brewton, R.G.; Wright, D.W.; Ren, Z.X. Morphological and biochemical studies of the structure of the vitreous and the zonular fibres. Biochem. Soc. Trans. 1991, 19, 868–871. [Google Scholar] [CrossRef]
- Ivanova, T.; Jalil, A.; Antoniou, Y.; Bishop, P.; Vallejo-Garcia, J.L.; Patton, N. Vitrectomy for primary symptomatic vitreous opacities: An evidence-based review. Eye 2016, 30, 645–655. [Google Scholar] [CrossRef]
- Schulz-Key, S.; Carlsson, J.O.; Crafoord, S. Long term follow-up of pars plana vitrectomy for vitreous floaters: Complications, outcomes and patient satisfaction. Acta Ophthalmol. 2011, 89, 159–165. [Google Scholar] [CrossRef]
- Sebag, J. Floaters and the Quality of Life. Am. J. Ophthalmol. 2011, 152, 3–4. [Google Scholar] [CrossRef]
- Wagle, A.M.; Lim, W.Y.; Yap, T.P.; Neelam, K.; Au Eong, K.G. Utility values associated with vitreous floaters. Am. J. Ophthalmol. 2011, 152, 60–65. [Google Scholar] [CrossRef]
- De Nie, K.F.; Crama, N.; Tilanus, M.A.; Klevering, B.J.; Boon, C.J. Pars plana vitrectomy for disturbing primary vitreous floaters: Clinical outcome and patient satisfaction. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1373–1382. [Google Scholar] [CrossRef]
- Ryan, E.H.; Lam, L.A.; Pulido, C.M.; Bennett, S.R.; Calabrese, A. Reading Speed as an Objective Measure of Improvement Following Vitrectomy for Symptomatic Vitreous Opacities. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 456–466. [Google Scholar] [CrossRef]
- Walton, K.A.; Meyer, C.H.; Harkrider, C.J.; Cox, T.A.; Toth, C.A. Age-Related Changes in Vitreous Mobility as Measured by Video B Scan Ultrasound. Exp. Eye Res. 2002, 74, 173–180. [Google Scholar] [CrossRef]
- Mamou, J.; Wa, C.A.; Yee, K.M.P.; Silverman, R.H.; Ketterling, J.; Sadun, A.A.; Sebag, J. Ultrasound-Based Quantification of Vitreous Floaters Correlates with Contrast Sensitivity and Quality of Life. Investig. Opthalmol Vis. Sci. 2015, 56, 1611–1617. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Nguyen-Cuu, J.; Yu, F.; Yee, K.M.; Mamou, J.; Silverman, R.H.; Ketterling, J.; Sebag, J. Assessment of Vitreous Structure and Visual Function after Neodymium:Yttrium–Aluminum–Garnet Laser Vitreolysis. Ophthalmology 2019, 126, 1517–1526. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Nguyen-Cuu, J.; Mamou, J.; Routledge, B.; Yee, K.M.; Sebag, J. Vitreous Structure and Visual Function in Myopic Vitreopathy Causing Vision-Degrading Myodesopsia. Am. J. Ophthalmol. 2021, 224, 246–253. [Google Scholar] [CrossRef]
- Stanga, P.E.; Sala-Puigdollers, A.; Caputo, S.; Jaberansari, H.; Cien, M.; Gray, J.; D’Souza, Y.; Charles, S.J.; Biswas, S.; Henson, D.B.; et al. In Vivo Imaging of Cortical Vitreous Using 1050-nm Swept-Source Deep Range Imaging Optical Coherence Tomography. Am. J. Ophthalmol. 2014, 157, 397–404. [Google Scholar] [CrossRef]
- Mojana, F.; Kozak, I.; Oster, S.F.; Cheng, L.; Bartsch, D.-U.G.; Brar, M.; Yuson, R.M.; Freeman, W.R. Observations by Spectral-Domain Optical Coherence Tomography Combined with Simultaneous Scanning Laser Ophthalmoscopy: Imaging of the Vitreous. Am. J. Ophthalmol. 2010, 149, 641–650. [Google Scholar] [CrossRef]
- Tsukahara, M.; Mori, K.; Gehlbach, P.L.; Mori, K. Posterior Vitreous Detachment as Observed by Wide-Angle OCT Imaging. Ophthalmology 2018, 125, 1372–1383. [Google Scholar] [CrossRef]
- Kraker, J.A.; Kim, J.E.; Koller, E.C.; George, J.C.; Hwang, E.S. Standard 6-mm Compared with Widefield 16.5-mm OCT for Staging of Posterior Vitreous Detachment. Ophthalmol. Retin. 2020, 4, 1093–1102. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W.; Pomerantzeff, O. Flying spot TV ophthalmoscope. Appl. Opt. 1980, 19, 2991–2997. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W. Scanning laser ophthalmoscope. IEEE Trans. Biomed. Eng. 1981, 28, 488–492. [Google Scholar] [CrossRef]
- Fischer, J.; Otto, T.; Delori, F.; Pace, L.; Staurenghi, G. Scanning Laser Ophthalmoscopy (SLO). In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]. Cham (CH); Springer: Manhattan, NY, USA, 2019; Chapter 2. [Google Scholar]
- Webb, R.H.; Hughes, G.W.; Delori, F.C. Confocal scanning laser ophthalmoscope. Appl. Opt. 1987, 26, 1492–1499. [Google Scholar] [CrossRef]
- Kruse, F.E.; Burk, R.O.; Völcker, H.-E.; Zinser, G.; Harbarth, U. Reproducibility of Topographic Measurements of the Optic Nerve Head with Laser Tomographic Scanning. Ophthalmology 1989, 96, 1320–1324. [Google Scholar] [CrossRef]
- Ryan, E.H.; Hassan, T.S.; Houston, S.K.S.; Kitchens, J.W.; Pollack, J.; Weng, C.Y. Vitreous Opacities. Current Trends and Treatment Strategies. Available online: https://retinatoday.com/articles/2021-jan-feb-supplement/vitreous-opacities (accessed on 20 October 2021).
- Garcia, G.A.; Khoshnevis, M.; Yee, K.M.P.; Nguyen-Cuu, J.; Nguyen, J.H.; Sebag, J. Degradation of Contrast Sensitivity Function Following Posterior Vitreous Detachment. Am. J. Ophthalmol. 2016, 172, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Vandorselaer, T.; Van De Velde, F.; Tassignon, M.J. Eligibility criteria for Nd-YAG laser treatment of highly symptomatic vitreous floaters. Bull. Soc. Belg. d’ophtalmologie 2001, 280, 15–19. [Google Scholar]
- Shaimova, V.A.; Shaimov, T.B.; Shaimov, R.B.; Galin, A.Y.; Goloshchapova, Z.A.; Ryzhkov, P.K.; Fomin, A.V. Evaluation of YAG-laser vitreolysis effectiveness based on quantitative characterization of vitreous floaters. Vestn. Oftalmol. 2018, 134, 56–62. [Google Scholar] [CrossRef]
- Sun, X.; Tian, J.; Wang, J.; Zhang, J.; Wang, Y.; Yuan, G. Nd:YAG Laser Vitreolysis for Symptomatic Vitreous Floaters: Application of Infrared Fundus Photography in Assessing the Treatment Efficacy. J. Ophthalmol. 2019, 2019, 8956952. [Google Scholar] [CrossRef]
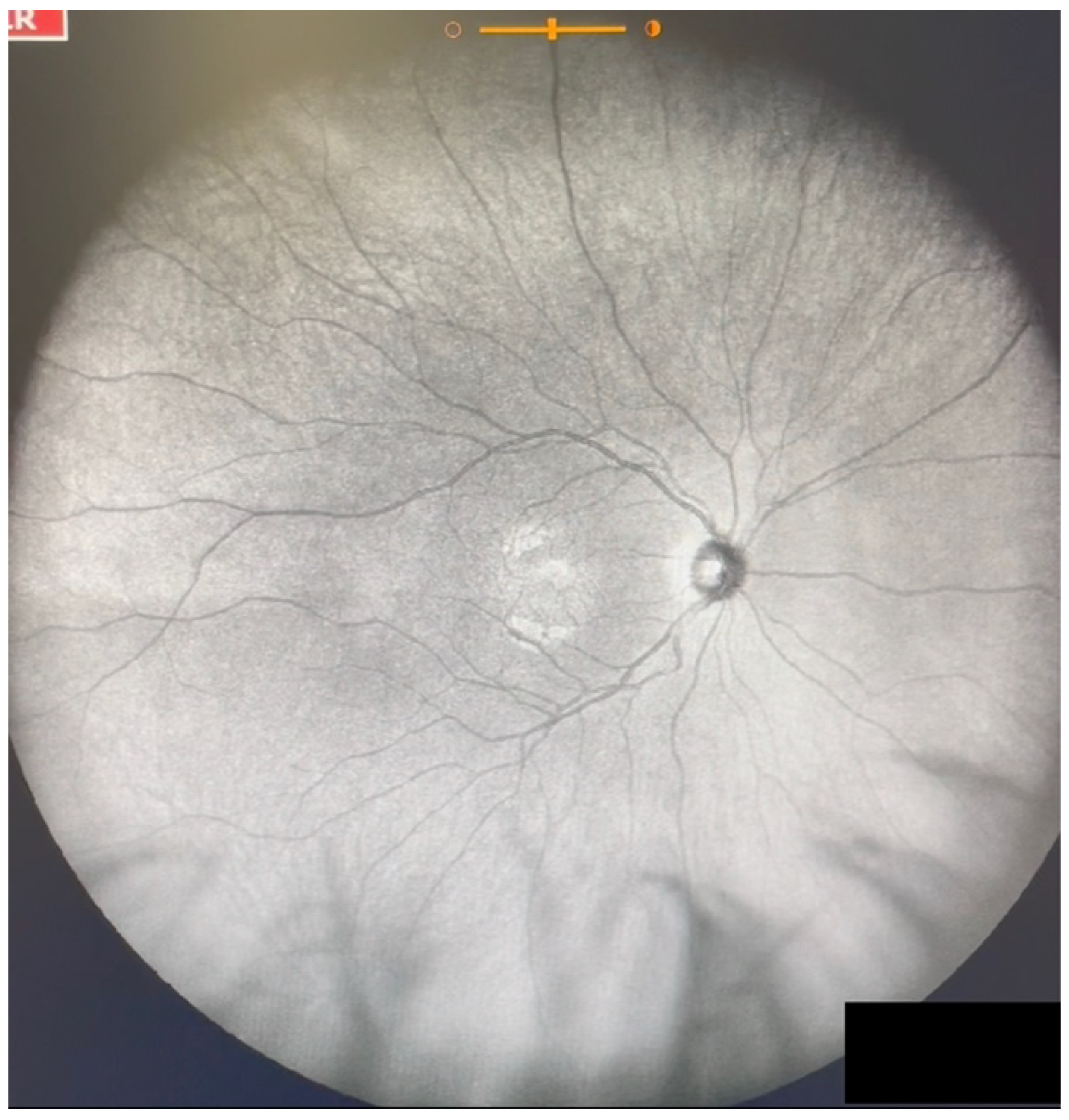
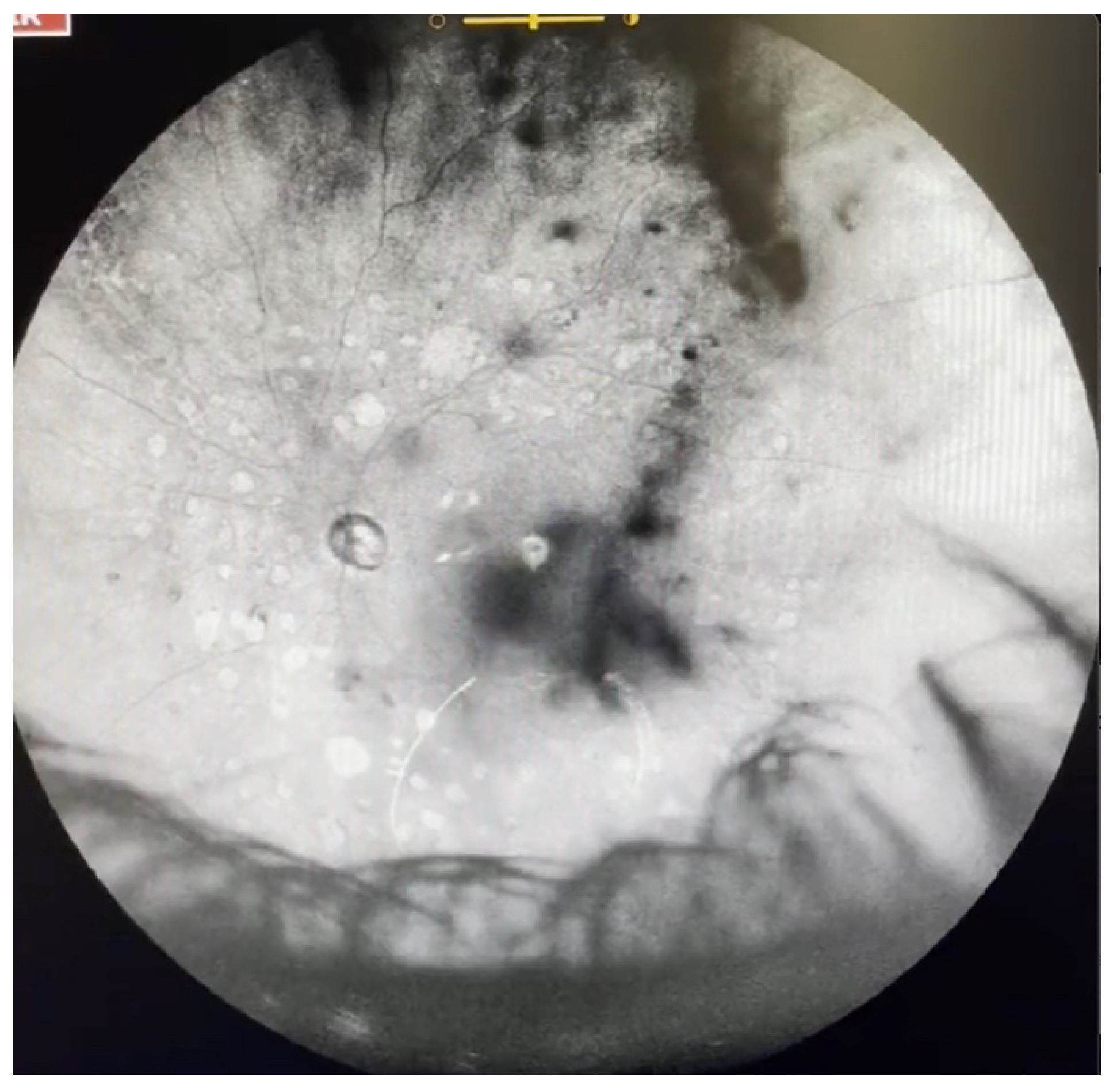
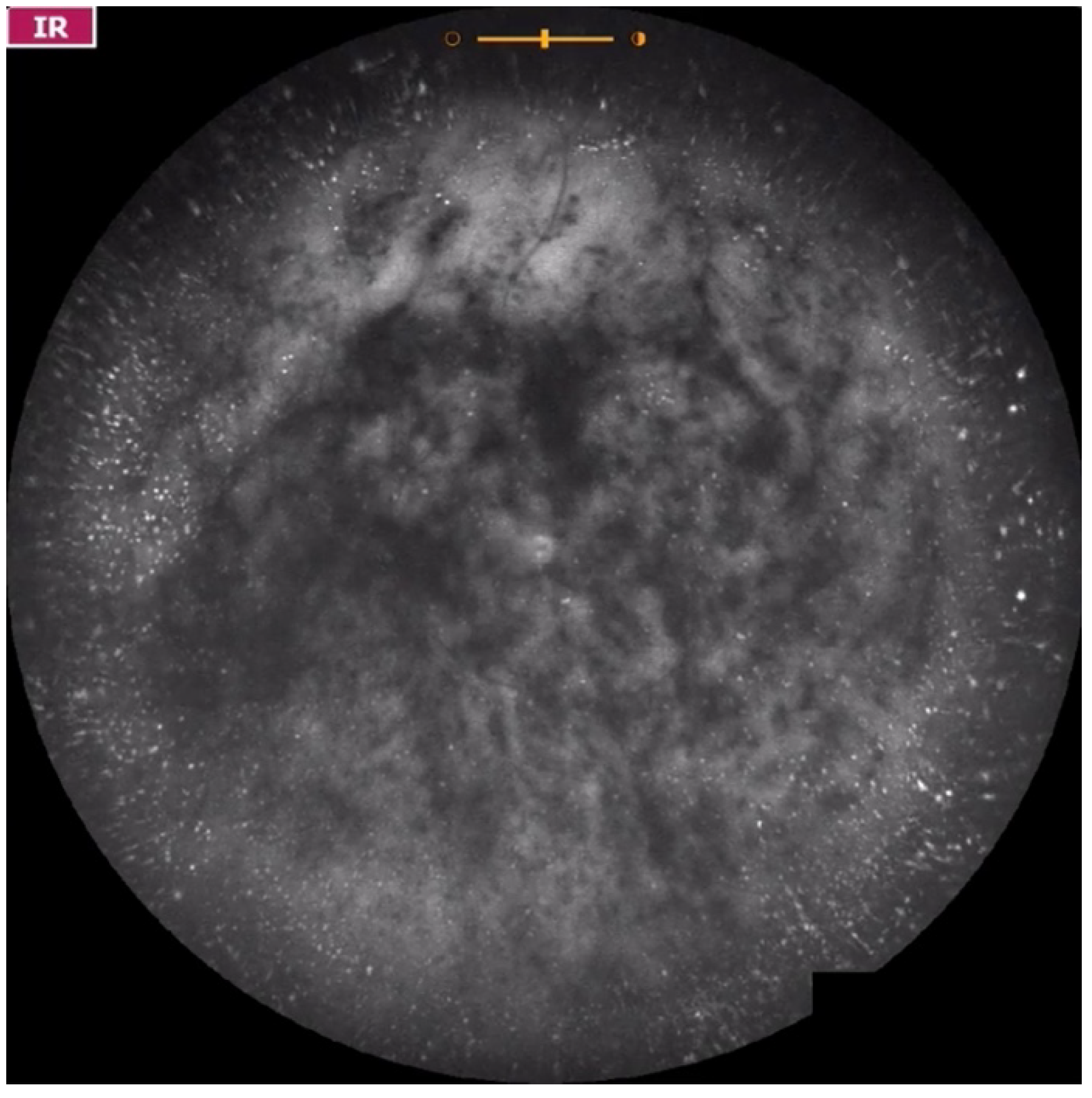
| Grade 0—No vitreous opacities visible (Figure 1 and Video S1). |
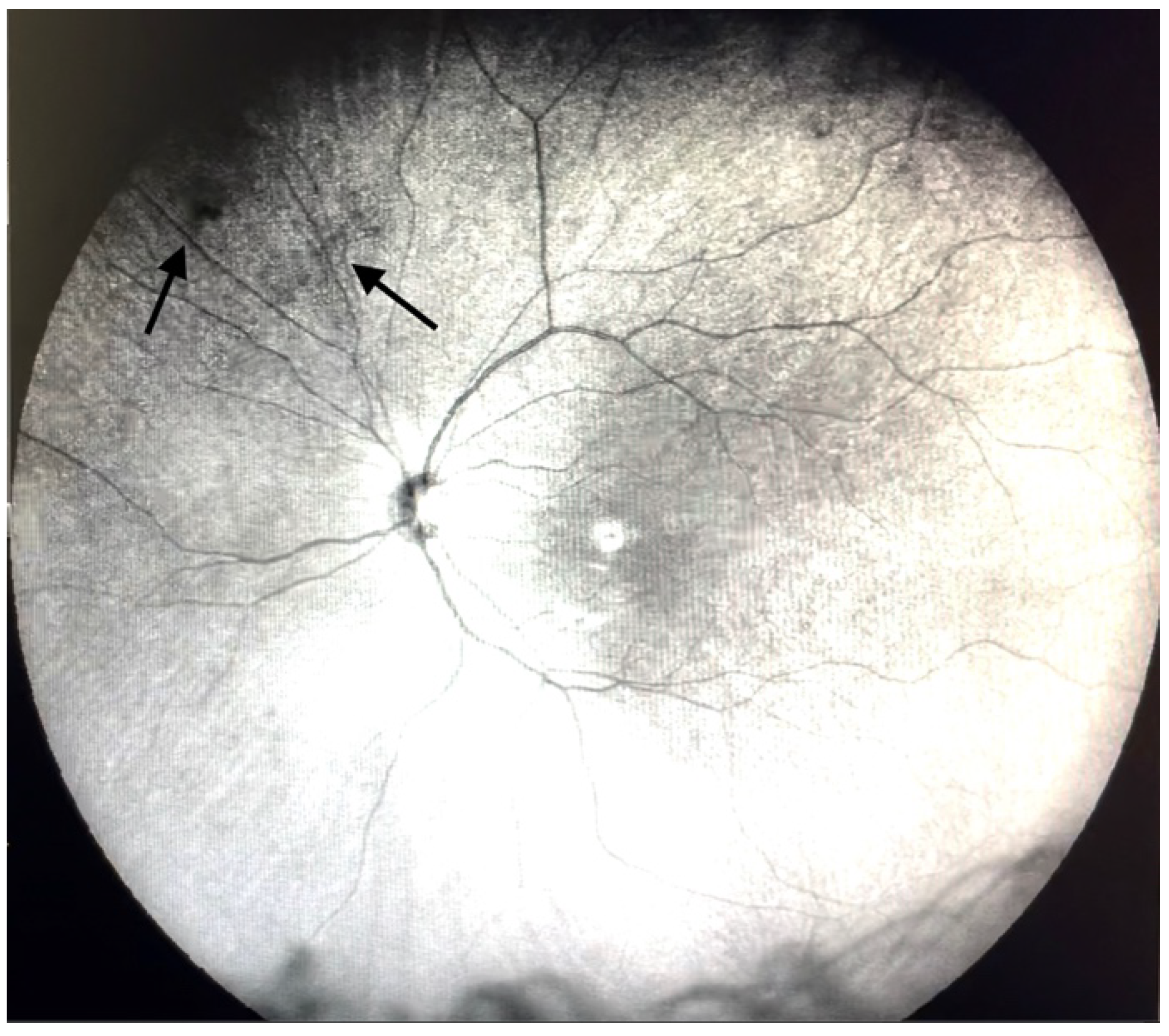
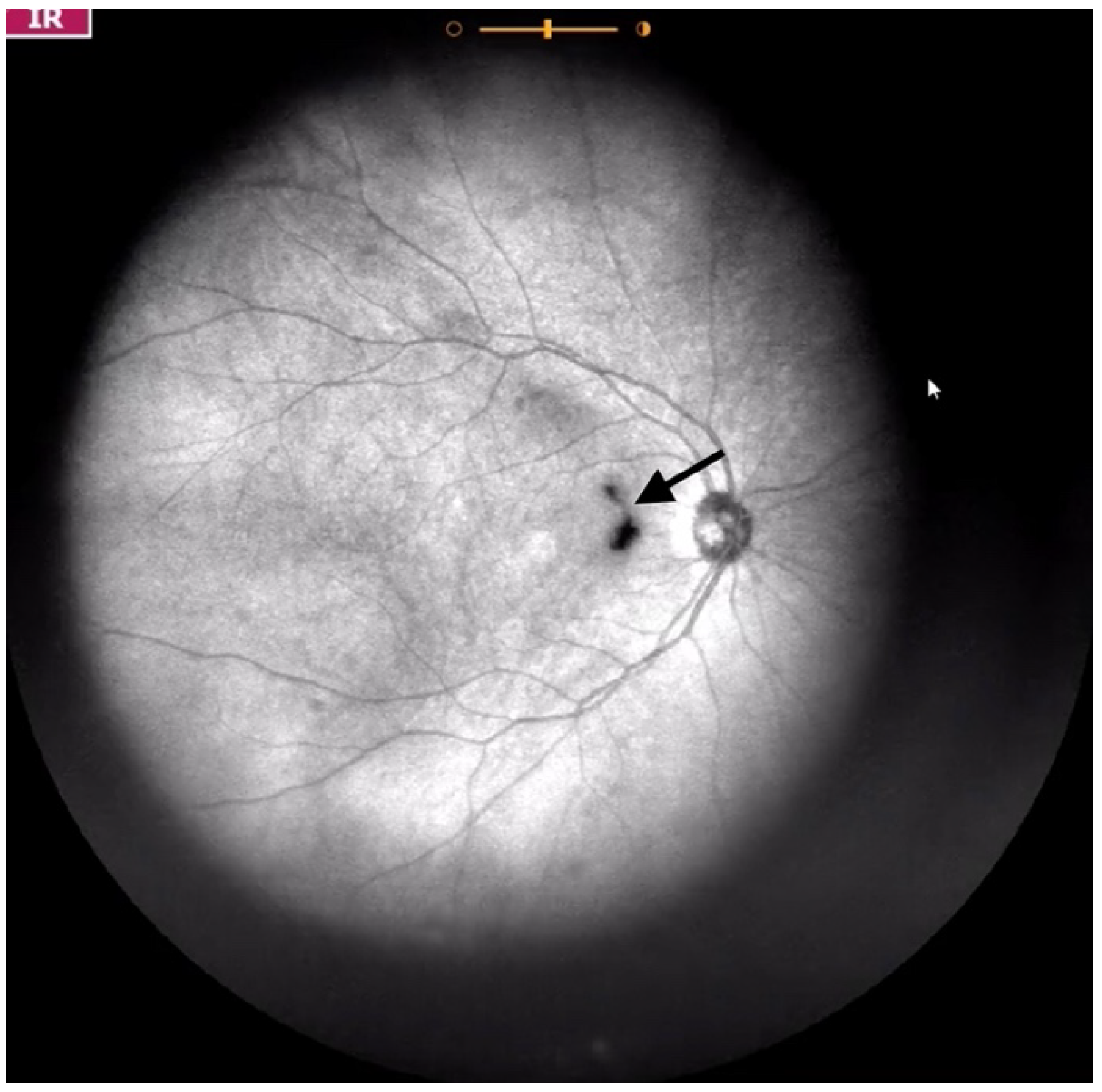
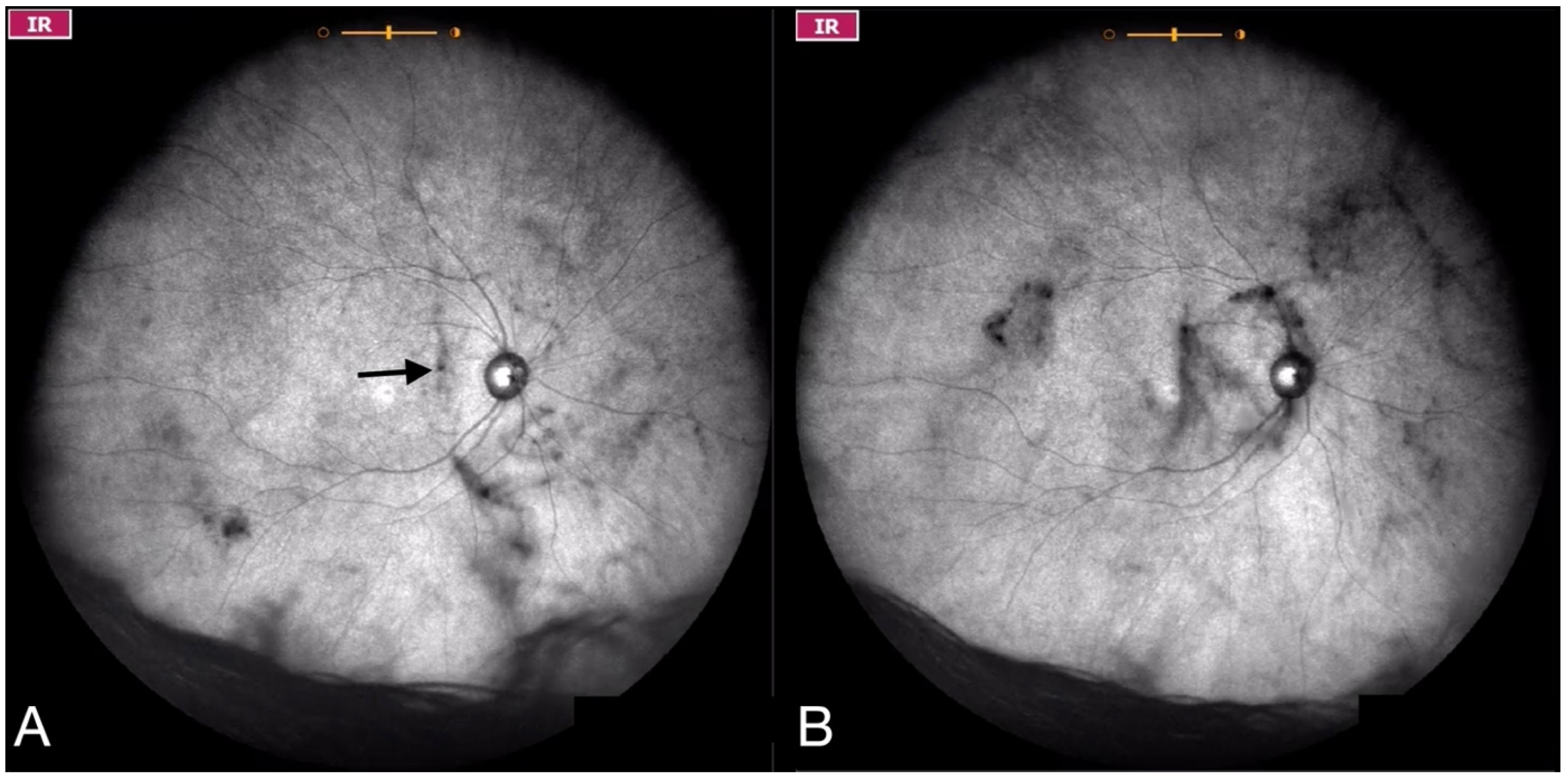
| Grade 1—Diffuse opacities that do not cross the center of the macula (Figure 2 and Video S2). |
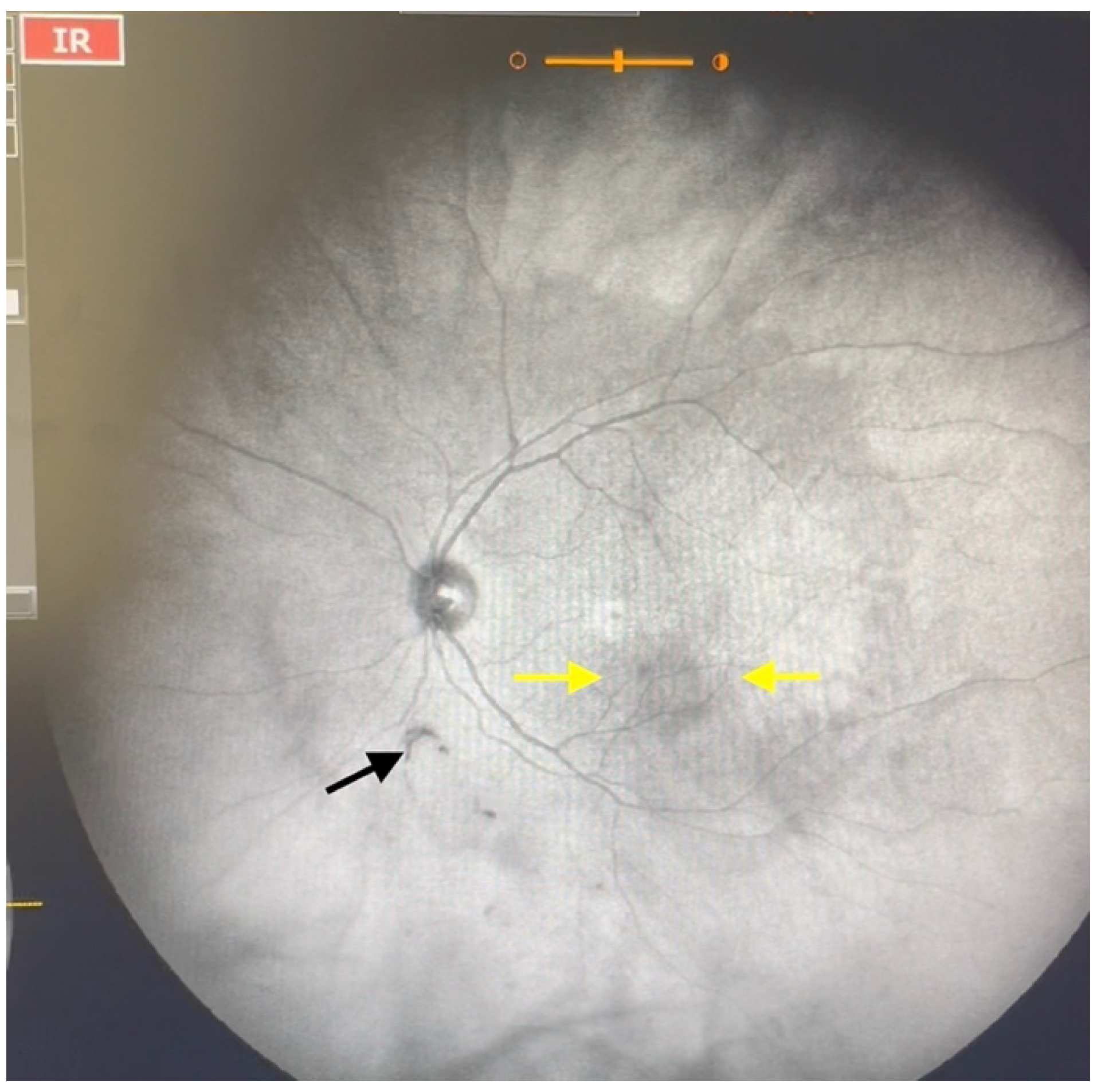
| Grade 2—Diffuse opacities that cross the center of the macula (Figure 3 and Video S3) and/or dense opacities that do not cross the center of the macula (Figure 4 and Video S4). |
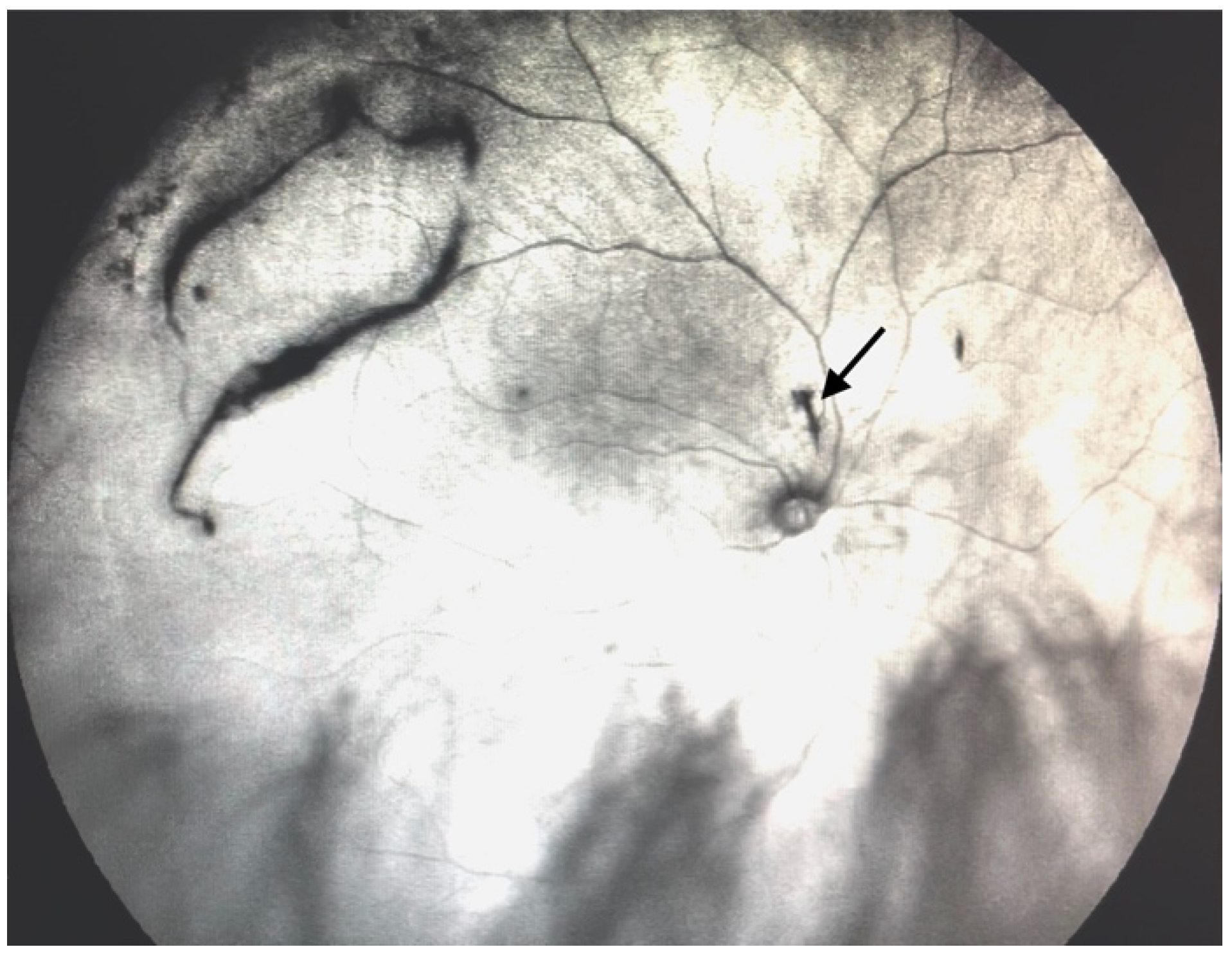
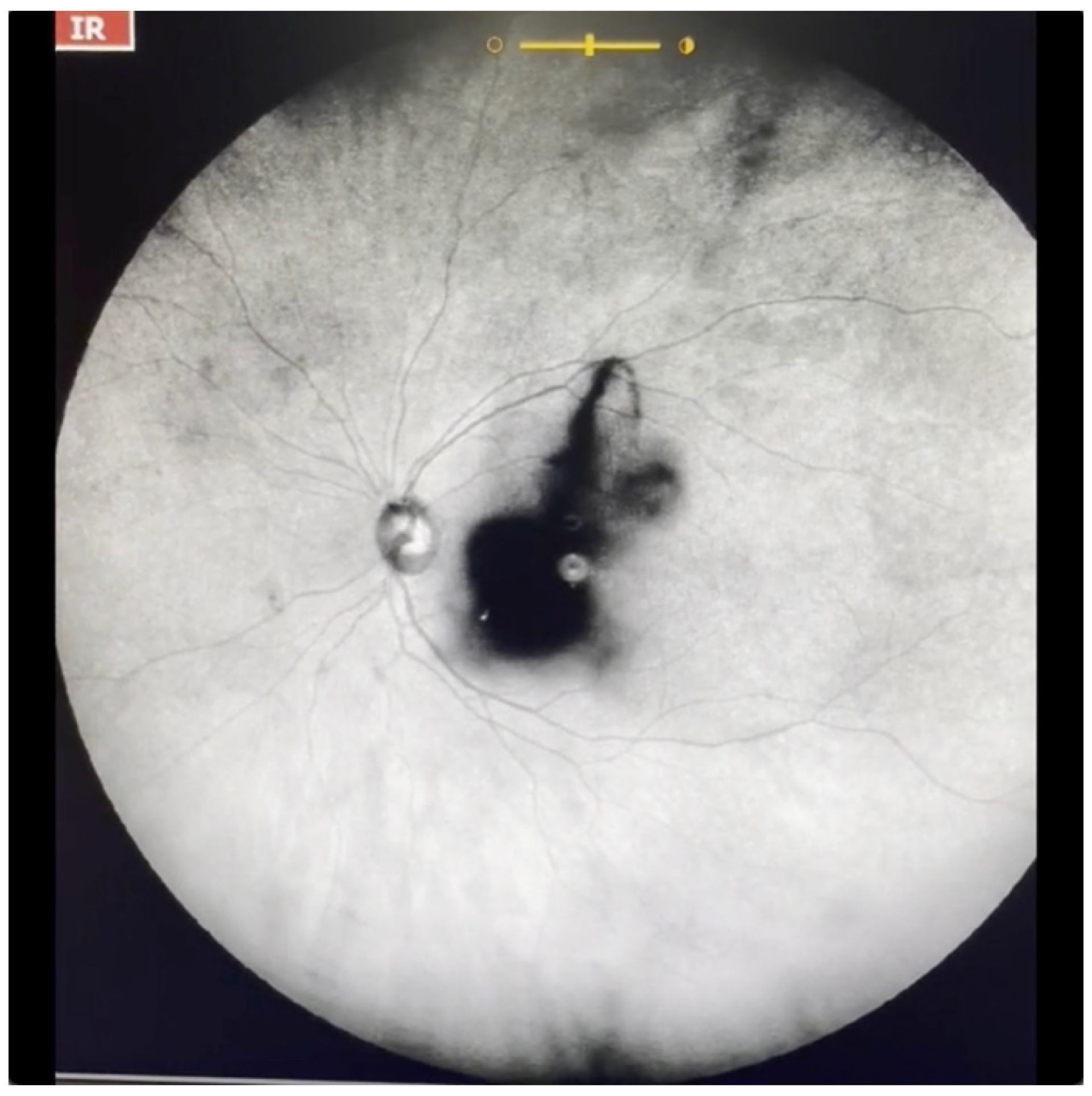
| Grade 3—Dense opacities outside the center of the macula in primary gaze, that cross the center of the macula with eye movement (Figure 5 and Video S5). |
| Grade 4—Dense opacities that involve the center of the macula in the primary gaze (Figure 6 and Video S6). |
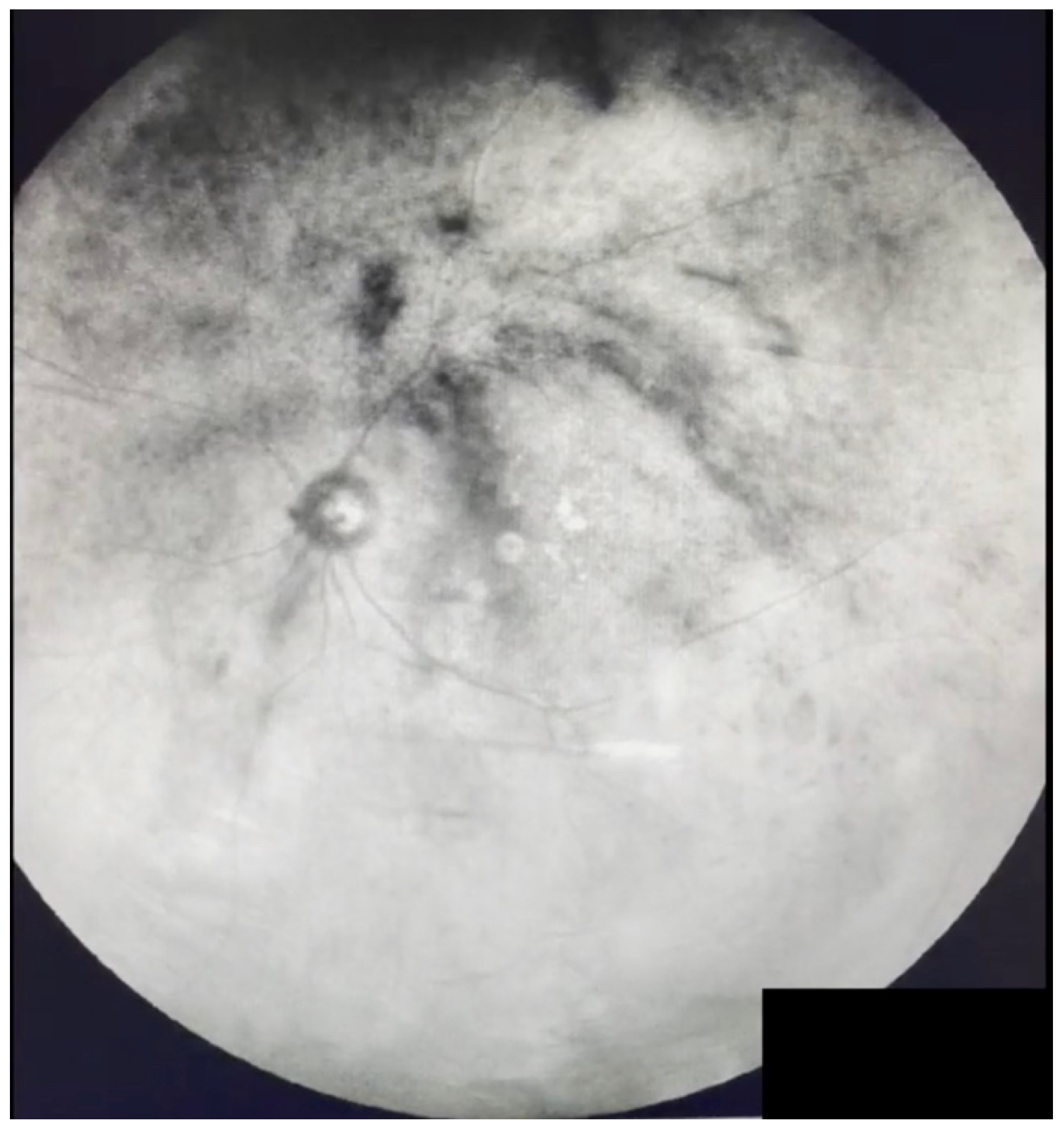
| Grade 5—Dense opacities that obstruct at least 30% of the macula (~2 disc diameters in size) (Figure 7 and Video S7). |
| Patient Number | Gender | Age (Years) | Eye | Time from Beginning of Symptoms (Days) | Severity of Symptoms | Diagnosis | Visible Weiss Ring | PVD per Structural OCT | Vitreous Opacities Severity Scale | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 39 | OD | 180 | + | Myopic vitreopathy | NO | NO | 2 | High myopia |
| OS | 180 | + | Myopic vitreopathy | NO | NO | 1 | High myopia | |||
| 2 | F | 25 | OD | 365 | + | Syneresis | NO | NO | 1 | |
| 3 | F | 24 | OS | 10 | + | Syneresis | NO | NO | 1 | |
| 4 | M | 70 | OD | >365 | + | Asteroid hyalosis | NO | NO | 2 | Asteroid hyalosis |
| OS | 1 | ++ | PVD | YES | YES | 2 | ||||
| 5 | F | 32 | OS | 60 | ++ | Myopic vitreopathy | NO | NO | 2 | High myopia |
| 6 | M | 59 | OD | >365 | + | Asteroid hyalosis | NO | NO | 2 | Asteroid hyalosis |
| OS | >365 | +++ | Asteroid hyalosis | NO | N/A | 5 | Asteroid hyalosis | |||
| 7 | M | 62 | OD | >365 | ++ | PVD | YES | YES | 3 | |
| OS | >365 | ++ | PVD | YES | YES | 2 | ||||
| 8 | F | 59 | OD | >365 | ++ | PVD | YES | YES | 2 | Peripheral lattice degeneration |
| 9 | M | 61 | OS | 30 | ++ | PVD | YES | YES | 2 | |
| 10 | M | 55 | OD | 120 | ++ | PVD | YES | YES | 3 | |
| OS | 120 | + | Syneresis | NO | NO | 2 | ||||
| 11 | M | 60 | OD | 180 | + | Syneresis | NO | NO | 2 | |
| OS | 180 | ++ | PVD | YES | YES | 3 | ||||
| 12 | M | 64 | OS | 4 | ++ | PVD | YES | YES | 3 | Diabetic macular edema |
| 13 | M | 64 | OD | 90 | + | PVD | YES | YES | 3 | Cataract |
| OS | 21 | ++ | PVD | YES | YES | 3 | Cataract | |||
| 14 | M | 47 | OD | 11 | ++ | PVD | YES | YES | 3 | |
| 15 | M | 66 | OD | 330 | ++ | PVD | YES | YES | 3 | |
| 16 | M | 56 | OD | 7 | +++ | PVD | YES | YES | 4 | |
| OS | 90 | ++ | PVD | YES | YES | 3 | ||||
| 17 | F | 69 | OD | 120 | ++ | PVD | YES | YES | 3 | |
| OS | 60 | ++ | PVD | YES | YES | 3 | ||||
| 18 | M | 58 | OS | 270 | ++ | PVD | YES | YES | 4 | |
| 19 | F | 62 | OS | 10 | +++ | Hemorrhagic PVD | NO | YES | 5 | |
| 20 | M | 81 | OS | 5 | +++ | Hemorrhagic PVD | NO | YES | 5 | Central retinal vein occlusion |
| 21 | M | 63 | OS | >365 | +++ | PVD | NO | YES | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Aguirre, G.; Henaine-Berra, A.; Salcedo-Villanueva, G. Visualization and Grading of Vitreous Floaters Using Dynamic Ultra-Widefield Infrared Confocal Scanning Laser Ophthalmoscopy: A Pilot Study. J. Clin. Med. 2022, 11, 5502. https://doi.org/10.3390/jcm11195502
Garcia-Aguirre G, Henaine-Berra A, Salcedo-Villanueva G. Visualization and Grading of Vitreous Floaters Using Dynamic Ultra-Widefield Infrared Confocal Scanning Laser Ophthalmoscopy: A Pilot Study. Journal of Clinical Medicine. 2022; 11(19):5502. https://doi.org/10.3390/jcm11195502
Chicago/Turabian StyleGarcia-Aguirre, Gerardo, Andree Henaine-Berra, and Guillermo Salcedo-Villanueva. 2022. "Visualization and Grading of Vitreous Floaters Using Dynamic Ultra-Widefield Infrared Confocal Scanning Laser Ophthalmoscopy: A Pilot Study" Journal of Clinical Medicine 11, no. 19: 5502. https://doi.org/10.3390/jcm11195502
APA StyleGarcia-Aguirre, G., Henaine-Berra, A., & Salcedo-Villanueva, G. (2022). Visualization and Grading of Vitreous Floaters Using Dynamic Ultra-Widefield Infrared Confocal Scanning Laser Ophthalmoscopy: A Pilot Study. Journal of Clinical Medicine, 11(19), 5502. https://doi.org/10.3390/jcm11195502






